Members involved:
Frédéric LEMAITRE, Manon GUILLE-COLLIGNON, Jérôme DELACOTTE, Olivier BURIEZ, Eric LABBE
(See our publications below)
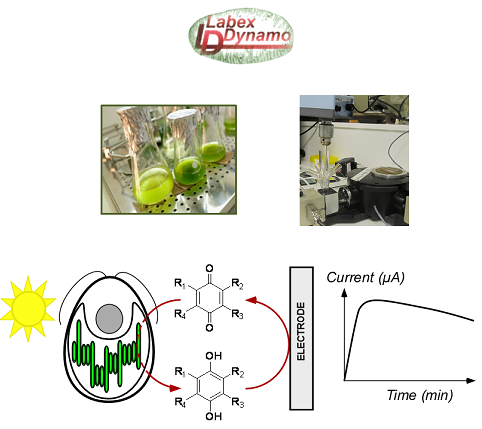
This project is devoted to the electrochemically-assisted diverting of electrons from photosynthesis. It notably aims at maintaining the photosynthetic efficiency under relatively high light conditions by the mean of electrochemical techniques for analytical (understanding of involved mechanisms) and preparative (dioxygen production / carbon dioxide conversion, plant cell lifetime) purposes. Indeed, the intrinsic saturation of photosynthesis leads to the formation of reactive species which may damage the photosynthetic chain. A relevant strategy thus consists in working with intact biological systems like algae and rerouting the overflow of electrons as an amenable photocurrent through the use of a collecting electrode/redox mediator tandem. However, this objective needs a tight compromise between the extraction yield and the incident light, i.e. minimizing the photoinhibition and maintaining algae alive because the photosynthetic electron extraction should lead to short-circuit the photosynthetic chain if maximizing the produced current. In this respect, many sub-strategies have to be developed. First of all, adapted mutants algae can be designed for a facilitated access to the extraction site for the redox mediator. Moreover, another critical issue is the nature of the electron carrier. Quinones look appropriate for such a purpose but they may act as “double agents”, i.e. they readily interact with the photosynthetic chain but also behave as poisons at long times. Therefore, designing functionalized quinones and new bio-inspired mediators are also considered. It therefore requires that structure-activity relationship of the exogenous quinones is better understood.
Our publications:
2019 |
Diverting photosynthetic electrons from suspensions of Chlamydomonas reinhardtii algae - New insights using an electrochemical well device Article de journal A Sayegh; G Longatte; O Buriez; F -A Wollman; M Guille-Collignon; E Labbé; J Delacotte; F Lemaître Electrochimica Acta, 304 , p. 465 - 473, 2019, ISSN: 0013-4686. @article{SAYEGH2019465, title = {Diverting photosynthetic electrons from suspensions of Chlamydomonas reinhardtii algae - New insights using an electrochemical well device}, author = {A Sayegh and G Longatte and O Buriez and F -A Wollman and M Guille-Collignon and E Labb\'{e} and J Delacotte and F Lema\^{i}tre}, url = {http://www.sciencedirect.com/science/article/pii/S0013468619303718}, doi = {https://doi.org/10.1016/j.electacta.2019.02.105}, issn = {0013-4686}, year = {2019}, date = {2019-01-01}, journal = {Electrochimica Acta}, volume = {304}, pages = {465 - 473}, abstract = {In the last years, many strategies have been developed to benefit from oxygenic photosynthesis in the present context of renewable energies. To achieve this, bioelectricity may be produced by using photosynthetic components involved in anodic or cathodic compartments. In this respect, harvesting photosynthetic electrons from living biological systems appears to be an encouraging approach. However it raises the question of the most suitable electrochemical device. In this work, we describe and analyze the performances of an electrochemical device based on a millimeter sized well involving a gold surface as a working electrode. Photocurrents were generated by suspensions of Chlamydomonas reinhardtii algae using quinones as mediators under different experimental conditions. Chronoamperometry and cyclic voltammetry measurements gave insight into the use of this device to investigate important issues (harvesting and poisoning by quinones, photoinactivation…). Furthermore, by introducing a kinetic model originally developed for homogeneous catalytic systems, the kinetics of the electron diverting from this system (Chlamydomonas reinhardtii algae + 2,6-DCBQ + miniaturized setup) can be estimated. All these results demonstrate that this experimental configuration is suitable for future works devoted to the choice of the best parameters in terms of long lasting performances.}, keywords = {}, pubstate = {published}, tppubtype = {article} } In the last years, many strategies have been developed to benefit from oxygenic photosynthesis in the present context of renewable energies. To achieve this, bioelectricity may be produced by using photosynthetic components involved in anodic or cathodic compartments. In this respect, harvesting photosynthetic electrons from living biological systems appears to be an encouraging approach. However it raises the question of the most suitable electrochemical device. In this work, we describe and analyze the performances of an electrochemical device based on a millimeter sized well involving a gold surface as a working electrode. Photocurrents were generated by suspensions of Chlamydomonas reinhardtii algae using quinones as mediators under different experimental conditions. Chronoamperometry and cyclic voltammetry measurements gave insight into the use of this device to investigate important issues (harvesting and poisoning by quinones, photoinactivation…). Furthermore, by introducing a kinetic model originally developed for homogeneous catalytic systems, the kinetics of the electron diverting from this system (Chlamydomonas reinhardtii algae + 2,6-DCBQ + miniaturized setup) can be estimated. All these results demonstrate that this experimental configuration is suitable for future works devoted to the choice of the best parameters in terms of long lasting performances. |
2018 |
Investigation of photocurrents resulting from a living unicellular algae suspension with quinones over time Article de journal G Longatte; A Sayegh; J Delacotte; F Rappaport; F -A Wollman; M Guille-Collignon; F Lemaître Chemical Science, 9 (43), p. 8271–8281, 2018. @article{Longatte:2018, title = {Investigation of photocurrents resulting from a living unicellular algae suspension with quinones over time}, author = {G Longatte and A Sayegh and J Delacotte and F Rappaport and F -A Wollman and M Guille-Collignon and F Lema\^{i}tre}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85056308267&doi=10.1039%2fc8sc03058h&partnerID=40&md5=73d658b7ab313cc1a772ca28dc56aa2d}, doi = {10.1039/c8sc03058h}, year = {2018}, date = {2018-01-01}, journal = {Chemical Science}, volume = {9}, number = {43}, pages = {8271--8281}, abstract = {Plants, algae, and some bacteria convert solar energy into chemical energy by using photosynthesis. In light of the current energy environment, many research strategies try to benefit from photosynthesis in order to generate usable photobioelectricity. Among all the strategies developed for transferring electrons from the photosynthetic chain to an outer collecting electrode, we recently implemented a method on a preparative scale (high surface electrode) based on a Chlamydomonas reinhardtii green algae suspension in the presence of exogenous quinones as redox mediators. While giving rise to an interesting performance (10-60 μA cm-2) in the course of one hour, this device appears to cause a slow decrease of the recorded photocurrent. In this paper, we wish to analyze and understand this gradual fall in performance in order to limit this issue in future applications. We thus first show that this kind of degradation could be related to over-irradiation conditions or side-effects of quinones depending on experimental conditions. We therefore built an empirical model involving a kinetic quenching induced by incubation with quinones, which is globally consistent with the experimental data provided by fluorescence measurements achieved after dark incubation of algae in the presence of quinones. © 2018 The Royal Society of Chemistry.}, keywords = {}, pubstate = {published}, tppubtype = {article} } Plants, algae, and some bacteria convert solar energy into chemical energy by using photosynthesis. In light of the current energy environment, many research strategies try to benefit from photosynthesis in order to generate usable photobioelectricity. Among all the strategies developed for transferring electrons from the photosynthetic chain to an outer collecting electrode, we recently implemented a method on a preparative scale (high surface electrode) based on a Chlamydomonas reinhardtii green algae suspension in the presence of exogenous quinones as redox mediators. While giving rise to an interesting performance (10-60 μA cm-2) in the course of one hour, this device appears to cause a slow decrease of the recorded photocurrent. In this paper, we wish to analyze and understand this gradual fall in performance in order to limit this issue in future applications. We thus first show that this kind of degradation could be related to over-irradiation conditions or side-effects of quinones depending on experimental conditions. We therefore built an empirical model involving a kinetic quenching induced by incubation with quinones, which is globally consistent with the experimental data provided by fluorescence measurements achieved after dark incubation of algae in the presence of quinones. © 2018 The Royal Society of Chemistry. |
2017 |
Electrochemical Harvesting of Photosynthetic Electrons from Unicellular Algae Population at the Preparative Scale by Using 2,6-dichlorobenzoquinone Article de journal G Longatte; F Rappaport; F -A Wollman; M Guille-Collignon; F Lemaître Electrochimica Acta, 236 , p. 337–342, 2017. @article{Longatte:2017, title = {Electrochemical Harvesting of Photosynthetic Electrons from Unicellular Algae Population at the Preparative Scale by Using 2,6-dichlorobenzoquinone}, author = {G Longatte and F Rappaport and F -A Wollman and M Guille-Collignon and F Lema\^{i}tre}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85016504359&doi=10.1016%2fj.electacta.2017.03.124&partnerID=40&md5=d8a7614ce4f287a9f115d922ab5ee8f6}, doi = {10.1016/j.electacta.2017.03.124}, year = {2017}, date = {2017-01-01}, journal = {Electrochimica Acta}, volume = {236}, pages = {337--342}, abstract = {Oxygenic photosynthesis is the process used by plants, cyanobacteria or algae to convert the solar energy into a chemical one from the carbon dioxide reduction and water oxidation. In the past years, many strategies were implemented to take benefits from the overall low yield of this process to extract photosynthetic electrons and thus produce a sustainable photocurrent. In practice, electrochemical tools were involved and the principle of electrons harvestings was related to the step of electron transfer between the photosynthetic organism and a collecting electrode. In this context, works involving an algae population in suspension were rather scarce and rather focus on the grafting of the photosynthetic machinery at the electrode surface. Based on our previous works, we report here the implementation of an electrochemical set-up at the preparative scale to produce photocurrents. An algae suspension, i.e. an intact biological system to ensure culture and growth, was involved in presence of a centimeter-sized carbon gauze as the collecting electrode. The spectroelectrochemical cell contains 16 mL of suspension of a Chlamydomonas reinhardtii mutant with an appropriate mediator (2,6-DCBQ). Under these conditions, stable photocurrents were recorded over 1 h whose magnitude depends on the quinone concentration and the light illumination. © 2017 Elsevier Ltd}, keywords = {}, pubstate = {published}, tppubtype = {article} } Oxygenic photosynthesis is the process used by plants, cyanobacteria or algae to convert the solar energy into a chemical one from the carbon dioxide reduction and water oxidation. In the past years, many strategies were implemented to take benefits from the overall low yield of this process to extract photosynthetic electrons and thus produce a sustainable photocurrent. In practice, electrochemical tools were involved and the principle of electrons harvestings was related to the step of electron transfer between the photosynthetic organism and a collecting electrode. In this context, works involving an algae population in suspension were rather scarce and rather focus on the grafting of the photosynthetic machinery at the electrode surface. Based on our previous works, we report here the implementation of an electrochemical set-up at the preparative scale to produce photocurrents. An algae suspension, i.e. an intact biological system to ensure culture and growth, was involved in presence of a centimeter-sized carbon gauze as the collecting electrode. The spectroelectrochemical cell contains 16 mL of suspension of a Chlamydomonas reinhardtii mutant with an appropriate mediator (2,6-DCBQ). Under these conditions, stable photocurrents were recorded over 1 h whose magnitude depends on the quinone concentration and the light illumination. © 2017 Elsevier Ltd |
G Longatte; M Guille-Collignon; F Lemaître ChemPhysChem, 18 (19), p. 2643–2650, 2017. @article{Longatte:2017a, title = {Electrocatalytic Mechanism Involving Michaelis\textendashMenten Kinetics at the Preparative Scale: Theory and Applicability to Photocurrents from a Photosynthetic Algae Suspension With Quinones}, author = {G Longatte and M Guille-Collignon and F Lema\^{i}tre}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85028605961&doi=10.1002%2fcphc.201700351&partnerID=40&md5=0500ecaa88d980132140883a725bedfb}, doi = {10.1002/cphc.201700351}, year = {2017}, date = {2017-01-01}, journal = {ChemPhysChem}, volume = {18}, number = {19}, pages = {2643--2650}, abstract = {In the past years, many strategies have been implemented to benefit from oxygenic photosynthesis to harvest photosynthetic electrons and produce a significant photocurrent. Therefore, electrochemical tools were considered and have globally relied on the electron transfer(s) between the photosynthetic chain and a collecting electrode. In this context, we recently reported the implementation of an electrochemical set-up at the preparative scale to produce photocurrents from a Chlamydomonas reinhardtii algae suspension with an appropriate mediator (2,6-DCBQ) and a carbon gauze as the working electrode. In the present work, we wish to describe a mathematical modeling of the recorded photocurrents to better understand the effects of the experimental conditions on the photosynthetic extraction of electrons. In that way, we established a general model of an electrocatalytic mechanism at the preparative scale (that is, assuming a homogenous bulk solution at any time and a constant diffusion layer, both assumptions being valid under forced convection) in which the chemical step involves a Michaelis\textendashMenten-like behaviour. Dependences of transient and steady-state corresponding currents were analysed as a function of different parameters by means of zone diagrams. This model was tested to our experimental data related to photosynthesis. The corresponding results suggest that competitive pathways beyond photosynthetic harvesting alone should be taken into account. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim}, keywords = {}, pubstate = {published}, tppubtype = {article} } In the past years, many strategies have been implemented to benefit from oxygenic photosynthesis to harvest photosynthetic electrons and produce a significant photocurrent. Therefore, electrochemical tools were considered and have globally relied on the electron transfer(s) between the photosynthetic chain and a collecting electrode. In this context, we recently reported the implementation of an electrochemical set-up at the preparative scale to produce photocurrents from a Chlamydomonas reinhardtii algae suspension with an appropriate mediator (2,6-DCBQ) and a carbon gauze as the working electrode. In the present work, we wish to describe a mathematical modeling of the recorded photocurrents to better understand the effects of the experimental conditions on the photosynthetic extraction of electrons. In that way, we established a general model of an electrocatalytic mechanism at the preparative scale (that is, assuming a homogenous bulk solution at any time and a constant diffusion layer, both assumptions being valid under forced convection) in which the chemical step involves a Michaelis–Menten-like behaviour. Dependences of transient and steady-state corresponding currents were analysed as a function of different parameters by means of zone diagrams. This model was tested to our experimental data related to photosynthesis. The corresponding results suggest that competitive pathways beyond photosynthetic harvesting alone should be taken into account. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim |
Redesigning the QA binding site of Photosystem II allows reduction of exogenous quinones Article de journal H -Y Fu; D Picot; Y Choquet; G Longatte; A Sayegh; J Delacotte; M Guille-Collignon; F Lemaýtre; F Rappaport; F -A Wollman Nature Communications, 8 , 2017. @article{Fu:2017, title = {Redesigning the QA binding site of Photosystem II allows reduction of exogenous quinones}, author = {H -Y Fu and D Picot and Y Choquet and G Longatte and A Sayegh and J Delacotte and M Guille-Collignon and F Lema\'{y}tre and F Rappaport and F -A Wollman}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85030692080&doi=10.1038%2fncomms15274&partnerID=40&md5=d602745399324d5a5eb6c25bd2e5a7c7}, doi = {10.1038/ncomms15274}, year = {2017}, date = {2017-01-01}, journal = {Nature Communications}, volume = {8}, abstract = {Strategies to harness photosynthesis from living organisms to generate electrical power have long been considered, yet efficiency remains low. Here, we aimed to reroute photosynthetic electron flow in photosynthetic organisms without compromising their phototrophic properties. We show that 2,6-dimethyl-p-benzoquinone (DMBQ) can be used as an electron mediator to assess the efficiency of mutations designed to engineer a novel electron donation pathway downstream of the primary electron acceptor QA of Photosystem (PS) II in the green alga Chlamydomonas reinhardtii. Through the use of structural prediction studies and a screen of site-directed PSII mutants we show that modifying the environment of the QA site increases the reduction rate of DMBQ. Truncating the C-terminus of the PsbT subunit protruding in the stroma provides evidence that shortening the distance between QA and DMBQ leads to sustained electron transfer to DMBQ, as confirmed by chronoamperometry, consistent with a bypass of the natural QA7circ; to QB pathway.}, keywords = {}, pubstate = {published}, tppubtype = {article} } Strategies to harness photosynthesis from living organisms to generate electrical power have long been considered, yet efficiency remains low. Here, we aimed to reroute photosynthetic electron flow in photosynthetic organisms without compromising their phototrophic properties. We show that 2,6-dimethyl-p-benzoquinone (DMBQ) can be used as an electron mediator to assess the efficiency of mutations designed to engineer a novel electron donation pathway downstream of the primary electron acceptor QA of Photosystem (PS) II in the green alga Chlamydomonas reinhardtii. Through the use of structural prediction studies and a screen of site-directed PSII mutants we show that modifying the environment of the QA site increases the reduction rate of DMBQ. Truncating the C-terminus of the PsbT subunit protruding in the stroma provides evidence that shortening the distance between QA and DMBQ leads to sustained electron transfer to DMBQ, as confirmed by chronoamperometry, consistent with a bypass of the natural QA7circ; to QB pathway. |
2016 |
Mechanism and analyses for extracting photosynthetic electrons using exogenous quinones-what makes a good extraction pathway? Article de journal G Longatte; F Rappaport; F -A Wollman; M Guille-Collignon; F Lemaître Photochemical and Photobiological Sciences, 15 (8), p. 969–979, 2016. @article{Longatte:2016, title = {Mechanism and analyses for extracting photosynthetic electrons using exogenous quinones-what makes a good extraction pathway?}, author = {G Longatte and F Rappaport and F -A Wollman and M Guille-Collignon and F Lema\^{i}tre}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-84982703184&doi=10.1039%2fc6pp00076b&partnerID=40&md5=bf03d01f7c5b19ff550d5b9f76d49eb9}, doi = {10.1039/c6pp00076b}, year = {2016}, date = {2016-01-01}, journal = {Photochemical and Photobiological Sciences}, volume = {15}, number = {8}, pages = {969--979}, abstract = {Plants or algae take many benefits from oxygenic photosynthesis by converting solar energy into chemical energy through the synthesis of carbohydrates from carbon dioxide and water. However, the overall yield of this process is rather low (about 4% of the total energy available from sunlight is converted into chemical energy). This is the principal reason why recently many studies have been devoted to extraction of photosynthetic electrons in order to produce a sustainable electric current. Practically, the electron transfer occurs between the photosynthetic organism and an electrode and can be assisted by an exogenous mediator, mainly a quinone. In this regard, we recently reported on a method involving fluorescence measurements to estimate the ability of different quinones to extract photosynthetic electrons from a mutant of Chlamydomonas reinhardtii. In the present work, we used the same kind of methodology to establish a zone diagram for predicting the most suitable experimental conditions to extract photoelectrons from intact algae (quinone concentration and light intensity) as a function of the purpose of the study. This will provide further insights into the extraction mechanism of photosynthetic electrons using exogenous quinones. Indeed fluorescence measurements allowed us to model the capacity of photosynthetic algae to donate electrons to an exogenous quinone by considering a numerical parameter called "open center ratio" which is related to the Photosystem II acceptor redox state. Then, using it as a proxy for investigating the extraction of photosynthetic electrons by means of an exogenous quinone, 2,6-DCBQ, we suggested an extraction mechanism that was globally found consistent with the experimentally extracted parameters. © The Royal Society of Chemistry and Owner Societies 2016.}, keywords = {}, pubstate = {published}, tppubtype = {article} } Plants or algae take many benefits from oxygenic photosynthesis by converting solar energy into chemical energy through the synthesis of carbohydrates from carbon dioxide and water. However, the overall yield of this process is rather low (about 4% of the total energy available from sunlight is converted into chemical energy). This is the principal reason why recently many studies have been devoted to extraction of photosynthetic electrons in order to produce a sustainable electric current. Practically, the electron transfer occurs between the photosynthetic organism and an electrode and can be assisted by an exogenous mediator, mainly a quinone. In this regard, we recently reported on a method involving fluorescence measurements to estimate the ability of different quinones to extract photosynthetic electrons from a mutant of Chlamydomonas reinhardtii. In the present work, we used the same kind of methodology to establish a zone diagram for predicting the most suitable experimental conditions to extract photoelectrons from intact algae (quinone concentration and light intensity) as a function of the purpose of the study. This will provide further insights into the extraction mechanism of photosynthetic electrons using exogenous quinones. Indeed fluorescence measurements allowed us to model the capacity of photosynthetic algae to donate electrons to an exogenous quinone by considering a numerical parameter called "open center ratio" which is related to the Photosystem II acceptor redox state. Then, using it as a proxy for investigating the extraction of photosynthetic electrons by means of an exogenous quinone, 2,6-DCBQ, we suggested an extraction mechanism that was globally found consistent with the experimentally extracted parameters. © The Royal Society of Chemistry and Owner Societies 2016. |
