Members involved:
Fatma BEN TRAD, Olivier BURIEZ, Eric LABBE, Jérôme DELACOTTE, Frédéric LEMAITRE, Manon GUILLE-COLLIGNON
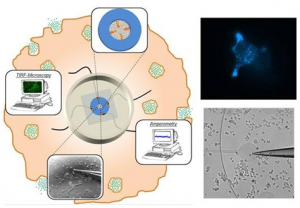
The cellular entrance and exit of bioactive molecules are key-issues for living organisms since they encompass crucial processes (cell signaling and communication, cellular excretory activity and drug delivery…) Whatever the nature of the uptake/release process, unsolved questions remain on the quantification of the species crossing the plasma membrane and on the description of the mechanistic steps involved at short timescales. Our project relies on the merging and coupling of electrochemical techniques (for the activation & detection of chemical species and for their time resolution) and fluorescence techniques (for their spatial sensitivity & imaging possibilities) to provide a better characterization of dynamic processes related to the internalization and the release of biomolecules – e.g. cell penetrating peptides & neurotransmitters. We propose an integrated approach where the quest for quantitative and time resolved information on transport governs both the design/preparation of adequate redox fluorescent probes and the conception of suitable setups and collecting/recording methodologies. This integrated “from the probe to the application” approach is aimed at making fluorescence and electrochemistry a designable, controllable and quantitative dual tool to explore trans-membrane traffic.
Our publications:
2019 |
Electroactive fluorescent false neurotransmitter FFN102 partially replaces dopamine in PC12 cell vesicles Article de journal L Hu; A Savy; L Grimaud; M Guille-Collignon; F Lemaître; C Amatore; J Delacotte Biophysical Chemistry, 245 , p. 1–5, 2019. @article{Hu:2019, title = {Electroactive fluorescent false neurotransmitter FFN102 partially replaces dopamine in PC12 cell vesicles}, author = {L Hu and A Savy and L Grimaud and M Guille-Collignon and F Lema\^{i}tre and C Amatore and J Delacotte}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85057201704&doi=10.1016%2fj.bpc.2018.11.001&partnerID=40&md5=28655b4c152ce0fc51fc037feefcff97}, doi = {10.1016/j.bpc.2018.11.001}, year = {2019}, date = {2019-01-01}, journal = {Biophysical Chemistry}, volume = {245}, pages = {1--5}, abstract = {In the last decade, following fluorescent dyes and protein tags, pH sensitive false fluorescent neurotransmitters (FFN) were introduced and were valuable for labeling secretory vesicles and monitoring exocytosis at living cells. In particular, the synthetic analog of neurotransmitters FFN102 was shown to be an electroactive probe. Here, we show that FFN102 is suitable to be used as a bioanalytic probe at the widely used PC12 cell model. FFN102 was uptaken in the secretory vesicles of PC12 cells, partially replacing the endogenous dopamine stored in these vesicles. The different oxidation potentials of dopamine and FFN102 allowed to determine that ca. 12% of dopamine was replaced by FFN102. Moreover, the FFN102 was found to be over released through the initial fusion pore suggesting that it was mostly uptaken in fast diffusion compartment of the vesicles. © 2018 Elsevier B.V.}, keywords = {}, pubstate = {published}, tppubtype = {article} } In the last decade, following fluorescent dyes and protein tags, pH sensitive false fluorescent neurotransmitters (FFN) were introduced and were valuable for labeling secretory vesicles and monitoring exocytosis at living cells. In particular, the synthetic analog of neurotransmitters FFN102 was shown to be an electroactive probe. Here, we show that FFN102 is suitable to be used as a bioanalytic probe at the widely used PC12 cell model. FFN102 was uptaken in the secretory vesicles of PC12 cells, partially replacing the endogenous dopamine stored in these vesicles. The different oxidation potentials of dopamine and FFN102 allowed to determine that ca. 12% of dopamine was replaced by FFN102. Moreover, the FFN102 was found to be over released through the initial fusion pore suggesting that it was mostly uptaken in fast diffusion compartment of the vesicles. © 2018 Elsevier B.V. |
2018 |
Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells Article de journal M Čížková; L Cattiaux; J Pandard; M Guille-Collignon; F Lemaître; J Delacotte; J -M Mallet; E Labbé; O Buriez Electrochemistry Communications, 97 , p. 46–50, 2018. @article{Cizkova:2018, title = {Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells}, author = {M \v{C}\'{i}\v{z}kov\'{a} and L Cattiaux and J Pandard and M Guille-Collignon and F Lema\^{i}tre and J Delacotte and J -M Mallet and E Labb\'{e} and O Buriez}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85054592456&doi=10.1016%2fj.elecom.2018.10.009&partnerID=40&md5=10a4aed1c89bb6a788a2a260bbd0a818}, doi = {10.1016/j.elecom.2018.10.009}, year = {2018}, date = {2018-01-01}, journal = {Electrochemistry Communications}, volume = {97}, pages = {46--50}, abstract = {An original redox-responsive fluorescent probe combining a rhodamine derivative and a ferrocenyl moiety used as the fluorescence modulator was designed, synthesized and characterized. The fluorescence of this new dyad could be tuned from the redox state of ferrocene, a feature observed both electrochemically and on cancer cells incubated with this probe. © 2018 Elsevier B.V.}, keywords = {}, pubstate = {published}, tppubtype = {article} } An original redox-responsive fluorescent probe combining a rhodamine derivative and a ferrocenyl moiety used as the fluorescence modulator was designed, synthesized and characterized. The fluorescence of this new dyad could be tuned from the redox state of ferrocene, a feature observed both electrochemically and on cancer cells incubated with this probe. © 2018 Elsevier B.V. |
X Liu; L Hu; N Pan; L Grimaud; E Labbé; O Buriez; J Delacotte; F Lemaître; M Guille-Collignon Biophysical Chemistry, 235 , p. 48–55, 2018. @article{Liu:2018, title = {Coupling electrochemistry and TIRF-microscopy with the fluorescent false neurotransmitter FFN102 supports the fluorescence signals during single vesicle exocytosis detection}, author = {X Liu and L Hu and N Pan and L Grimaud and E Labb\'{e} and O Buriez and J Delacotte and F Lema\^{i}tre and M Guille-Collignon}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85042352158&doi=10.1016%2fj.bpc.2018.02.004&partnerID=40&md5=365430d79a0d526895e755729264d88f}, doi = {10.1016/j.bpc.2018.02.004}, year = {2018}, date = {2018-01-01}, journal = {Biophysical Chemistry}, volume = {235}, pages = {48--55}, abstract = {Applications of the Fluorescent False Neurotransmitter FFN102, an analog of biogenic neurotransmitters and a suitable probe for coupled amperometry and TIRFM (total internal reflexion fluorescence microscopy) investigations of exocytotic secretion, were considered here. The electroactivity of FFN102 was shown to very likely arise from the oxidation of its phenolic group through a CE (Chemical-Electrochemical) mechanism. Evidences that the aminoethyl group of FFN102 is the key recognition element by BON N13 cells were also provided. Amperometric measurements were then performed at the single cell level with carbon fiber electrode (CFE) or Indium Tin Oxide (ITO) surfaces. It proved the disparity of kinetic and quantitative parameters of FFN102-stained cells acquired either at cell top and bottom. Moreover, coupled analyses of FFN102 loaded vesicles allowed us to classify three types of optical signals that probably arise from secretion releases thanks to their concomitant detection with an electrochemical spike. Finally, preliminary benefits from the coupling involving FFN102 were reported in terms of origins of overlapped amperometric spikes or assignment of fluorescence extinctions to real exocytotic events. © 2018 Elsevier B.V.}, keywords = {}, pubstate = {published}, tppubtype = {article} } Applications of the Fluorescent False Neurotransmitter FFN102, an analog of biogenic neurotransmitters and a suitable probe for coupled amperometry and TIRFM (total internal reflexion fluorescence microscopy) investigations of exocytotic secretion, were considered here. The electroactivity of FFN102 was shown to very likely arise from the oxidation of its phenolic group through a CE (Chemical-Electrochemical) mechanism. Evidences that the aminoethyl group of FFN102 is the key recognition element by BON N13 cells were also provided. Amperometric measurements were then performed at the single cell level with carbon fiber electrode (CFE) or Indium Tin Oxide (ITO) surfaces. It proved the disparity of kinetic and quantitative parameters of FFN102-stained cells acquired either at cell top and bottom. Moreover, coupled analyses of FFN102 loaded vesicles allowed us to classify three types of optical signals that probably arise from secretion releases thanks to their concomitant detection with an electrochemical spike. Finally, preliminary benefits from the coupling involving FFN102 were reported in terms of origins of overlapped amperometric spikes or assignment of fluorescence extinctions to real exocytotic events. © 2018 Elsevier B.V. |
Electrochemical switching fluorescence emission in rhodamine derivatives Article de journal M Čížková; L Cattiaux; J -M Mallet; E Labbé; O Buriez Electrochimica Acta, 260 , p. 589–597, 2018. @article{Cizkova:2018a, title = {Electrochemical switching fluorescence emission in rhodamine derivatives}, author = {M \v{C}\'{i}\v{z}kov\'{a} and L Cattiaux and J -M Mallet and E Labb\'{e} and O Buriez}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85038870042&doi=10.1016%2fj.electacta.2017.12.104&partnerID=40&md5=6a1537d7de8ad37549ef796b0c6f2642}, doi = {10.1016/j.electacta.2017.12.104}, year = {2018}, date = {2018-01-01}, journal = {Electrochimica Acta}, volume = {260}, pages = {589--597}, abstract = {Three rhodamine derivatives exhibiting electrofluorochromic properties were investigated by cyclic voltammetry and UV\textendashVis/fluorescence spectroelectrochemistry. Rhodamine 101 (Rh101, compound 1) was used as a reference model. In compound 2, the carboxylate anion of Rh101 was replaced by an alkyne moiety to allow further functionalization. The compound 3 was prepared from 2 by conversion of the alkyne to a triazole group bearing an alkyl chain with an alcohol function. These three rhodamine derivatives exhibited similar electrochemical behaviors. Their mono-electronic reductions produced the corresponding radical species which were stable on the time-scale of cyclic voltammetry. Additional reduction of electrogenerated radicals produced unstable anions which underwent subsequent chemical reaction, most likely protonation. Based on cyclic voltammetry investigations, absorption and fluorescence spectroelectrochemistry were then performed on compounds 1, 2, 3 and their parent reduced radicals 1a, 2a, 3a. UV\textendashVis spectroelectrochemistry, combined with TD-DFT calculation, confirmed the formation of radicals upon mono-electronic reduction of starting rhodamines. Fluorescence spectroelectrochemistry showed that, contrary to their parent molecules, electrogenerated radicals were non-fluorescent. Electrochemical fluorescence extinction was successfully achieved with all studied compounds. Moreover, compound 1 underwent on/off switching between fluorescent and non-fluorescent states repeatedly. Also, recovery of fluorescence in compound 3 was observed, which open interesting opportunities for the development of versatile rhodamine-based probes. © 2017 The Authors}, keywords = {}, pubstate = {published}, tppubtype = {article} } Three rhodamine derivatives exhibiting electrofluorochromic properties were investigated by cyclic voltammetry and UV–Vis/fluorescence spectroelectrochemistry. Rhodamine 101 (Rh101, compound 1) was used as a reference model. In compound 2, the carboxylate anion of Rh101 was replaced by an alkyne moiety to allow further functionalization. The compound 3 was prepared from 2 by conversion of the alkyne to a triazole group bearing an alkyl chain with an alcohol function. These three rhodamine derivatives exhibited similar electrochemical behaviors. Their mono-electronic reductions produced the corresponding radical species which were stable on the time-scale of cyclic voltammetry. Additional reduction of electrogenerated radicals produced unstable anions which underwent subsequent chemical reaction, most likely protonation. Based on cyclic voltammetry investigations, absorption and fluorescence spectroelectrochemistry were then performed on compounds 1, 2, 3 and their parent reduced radicals 1a, 2a, 3a. UV–Vis spectroelectrochemistry, combined with TD-DFT calculation, confirmed the formation of radicals upon mono-electronic reduction of starting rhodamines. Fluorescence spectroelectrochemistry showed that, contrary to their parent molecules, electrogenerated radicals were non-fluorescent. Electrochemical fluorescence extinction was successfully achieved with all studied compounds. Moreover, compound 1 underwent on/off switching between fluorescent and non-fluorescent states repeatedly. Also, recovery of fluorescence in compound 3 was observed, which open interesting opportunities for the development of versatile rhodamine-based probes. © 2017 The Authors |
2017 |
A Dual Functional Electroactive and Fluorescent Probe for Coupled Measurements of Vesicular Exocytosis with High Spatial and Temporal Resolution Article de journal X Liu; A Savy; S Maurin; L Grimaud; F Darchen; D Quinton; E Labbé; O Buriez; J Delacotte; F Lemaître; M Guille-Collignon Angewandte Chemie - International Edition, 56 (9), p. 2366–2370, 2017. @article{Liu:2017a, title = {A Dual Functional Electroactive and Fluorescent Probe for Coupled Measurements of Vesicular Exocytosis with High Spatial and Temporal Resolution}, author = {X Liu and A Savy and S Maurin and L Grimaud and F Darchen and D Quinton and E Labb\'{e} and O Buriez and J Delacotte and F Lema\^{i}tre and M Guille-Collignon}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85010696856&doi=10.1002%2fanie.201611145&partnerID=40&md5=a51767157166d7f185f0195a28b347b8}, doi = {10.1002/anie.201611145}, year = {2017}, date = {2017-01-01}, journal = {Angewandte Chemie - International Edition}, volume = {56}, number = {9}, pages = {2366--2370}, abstract = {In this work, Fluorescent False Neurotransmitter 102 (FFN102), a synthesized analogue of biogenic neurotransmitters, was demonstrated to show both pH-dependent fluorescence and electroactivity. To study secretory behaviors at the single-vesicle level, FFN102 was employed as a new fluorescent/electroactive dual probe in a coupled technique (amperometry and total internal reflection fluorescence microscopy (TIRFM)). We used N13 cells, a stable clone of BON cells, to specifically accumulate FFN102 into their secretory vesicles, and then optical and electrochemical measurements of vesicular exocytosis were experimentally achieved by using indium tin oxide (ITO) transparent electrodes. Upon stimulation, FFN102 started to diffuse out from the acidic intravesicular microenvironment to the neutral extracellular space, leading to fluorescent emissions and to the electrochemical oxidation signals that were simultaneously collected from the ITO electrode surface. The correlation of fluorescence and amperometric signals resulting from the FFN102 probe allows real-time monitoring of single exocytotic events with both high spatial and temporal resolution. This work opens new possibilities in the investigation of exocytotic mechanisms. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim}, keywords = {}, pubstate = {published}, tppubtype = {article} } In this work, Fluorescent False Neurotransmitter 102 (FFN102), a synthesized analogue of biogenic neurotransmitters, was demonstrated to show both pH-dependent fluorescence and electroactivity. To study secretory behaviors at the single-vesicle level, FFN102 was employed as a new fluorescent/electroactive dual probe in a coupled technique (amperometry and total internal reflection fluorescence microscopy (TIRFM)). We used N13 cells, a stable clone of BON cells, to specifically accumulate FFN102 into their secretory vesicles, and then optical and electrochemical measurements of vesicular exocytosis were experimentally achieved by using indium tin oxide (ITO) transparent electrodes. Upon stimulation, FFN102 started to diffuse out from the acidic intravesicular microenvironment to the neutral extracellular space, leading to fluorescent emissions and to the electrochemical oxidation signals that were simultaneously collected from the ITO electrode surface. The correlation of fluorescence and amperometric signals resulting from the FFN102 probe allows real-time monitoring of single exocytotic events with both high spatial and temporal resolution. This work opens new possibilities in the investigation of exocytotic mechanisms. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim |
R de Oliveira; M Durand; L Challier; P Messina; J M Swiecicki; M Di Pisa; G Chassaing; S Lavielle; O Buriez; E Labbé Journal of Electroanalytical Chemistry, 788 , p. 225–231, 2017. @article{deOliveira:2017, title = {Electrochemical quenching of the fluorescence produced by NBD-labelled cell penetrating peptides: A contribution to the study of their internalization in large unilamellar vesicles}, author = {R de Oliveira and M Durand and L Challier and P Messina and J M Swiecicki and M Di Pisa and G Chassaing and S Lavielle and O Buriez and E Labb\'{e}}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85013176758&doi=10.1016%2fj.jelechem.2017.02.006&partnerID=40&md5=725e8c07b1f4090ecf4f2ceabb57e6f7}, doi = {10.1016/j.jelechem.2017.02.006}, year = {2017}, date = {2017-01-01}, journal = {Journal of Electroanalytical Chemistry}, volume = {788}, pages = {225--231}, abstract = {This work investigates the implementation of a simple and versatile electrochemical setup aimed at achieving a fast and complete fluorescence extinction of NBD-labelled (NBD = 7-nitrobenz-2-oxa-1,3-diazole) cell penetrating peptides contained in 2\textendash5 cm3 samples containing phosphate buffer + large unilamellar vesicles. The quenching is obtained through a reductive electrolysis in a 2-compartment cell homebuilt from disposable plastic labware, which remains inert towards the adsorption of both peptides and lipid vesicles. Considering the micromolar concentration of NBD-tagged peptides, the main electrochemical reaction observed is hydrogen evolution, NBD reduction representing a small fraction of the cathodic current/charge engaged. The electrolysis conditions are discussed with respect to the nature of the reduction products formed, the integrity of large unilamellar vesicles and phosphate buffering properties. This electrochemical method is compared to the traditional chemical dithionite quenching of NBD and tested to monitor the internalization of cell penetrating peptides in large unilamellar vesicles. © 2017 Elsevier B.V.}, keywords = {}, pubstate = {published}, tppubtype = {article} } This work investigates the implementation of a simple and versatile electrochemical setup aimed at achieving a fast and complete fluorescence extinction of NBD-labelled (NBD = 7-nitrobenz-2-oxa-1,3-diazole) cell penetrating peptides contained in 2–5 cm3 samples containing phosphate buffer + large unilamellar vesicles. The quenching is obtained through a reductive electrolysis in a 2-compartment cell homebuilt from disposable plastic labware, which remains inert towards the adsorption of both peptides and lipid vesicles. Considering the micromolar concentration of NBD-tagged peptides, the main electrochemical reaction observed is hydrogen evolution, NBD reduction representing a small fraction of the cathodic current/charge engaged. The electrolysis conditions are discussed with respect to the nature of the reduction products formed, the integrity of large unilamellar vesicles and phosphate buffering properties. This electrochemical method is compared to the traditional chemical dithionite quenching of NBD and tested to monitor the internalization of cell penetrating peptides in large unilamellar vesicles. © 2017 Elsevier B.V. |
2015 |
Bioanalytical applications of the fluorescence-electrochemistry combination Article de journal F Lemaître; M Guille-Collignon Actualite Chimique, (400-401), p. 17–19, 2015. @article{Lemaitre:2015, title = {Bioanalytical applications of the fluorescence-electrochemistry combination}, author = {F Lema\^{i}tre and M Guille-Collignon}, url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-84973499998&partnerID=40&md5=d5fb99aaf2d3336a53a286546cb86bdb}, year = {2015}, date = {2015-01-01}, journal = {Actualite Chimique}, number = {400-401}, pages = {17--19}, abstract = {Both fluorescence and electrochemistry techniques aim at converting a chemical signal into an optical or an electrical one respectively. Particularly, they correspond to appropriate techniques for investigating biological phenomena due to the electroactivity of many biomolecules while cells or proteins can be labeled with fluorophores. Therefore, this article is a non exhaustive presentation of the coupling between electrochemistry and fluorescence for biological investigations. By focusing on exocytosis, it also raises the question of the implementation of such a combination.}, keywords = {}, pubstate = {published}, tppubtype = {article} } Both fluorescence and electrochemistry techniques aim at converting a chemical signal into an optical or an electrical one respectively. Particularly, they correspond to appropriate techniques for investigating biological phenomena due to the electroactivity of many biomolecules while cells or proteins can be labeled with fluorophores. Therefore, this article is a non exhaustive presentation of the coupling between electrochemistry and fluorescence for biological investigations. By focusing on exocytosis, it also raises the question of the implementation of such a combination. |
