Jean-Maurice Mallet (DR CNRS), Blaise Dumat (CR CNRS)
- Hybrid chemogenetic probes for imaging and sensing
-
- Fluorogenic HaloTag probes
We have developed a palette of fluorogenic probes targeting the protein self-labeling tag HaloTag for subcellular imaging of proteins and organelles. The probes are based on a versatile molecular rotor design that enables facile tuning of the photophysical and biophysical properties in order to tune the emission wavelength and optimize the selectivity.
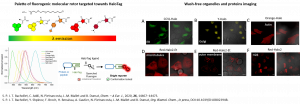
-
- Dual-Input functional probes
A second part of the project aims at coupling the fluorogens to a sensing group to design hybrid sensors for calcium or pH. The combination of the fluorogenic targeting of genetically-encoded proteins with the functional sensing ability should enable the selective imaging of the target analyte in genetically-defined organelles or cell types even in wash-free imaging conditions.
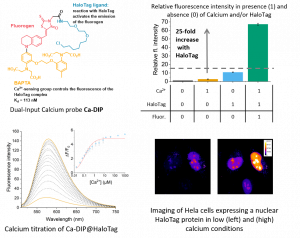
Funding: ANR JCJC DIPimaging (2019-2022, PI: Blaise Dumat)
Collaborations: N. Pietrancosta and A. Gautier (LBM), Vincent Vialou (Neurosciences Paris Seine)
Publications:
Bachollet, S. ; Pietrancosta, N.; Mallet, J.-M.; Dumat, B. Chem. Commun. 2022. doi:10.1039/D2CC01792J
S. Bachollet, Y. Shpinov, F. Broch, H. Benaissa, A. Gautier, N. Pietrancosta, J.-M. Mallet and B. Dumat, Org. Biomol. Chem., 2022, 20, 3619-3628
S. Bachollet, C. Addi, N. Pietrancosta, J.-M. Mallet and B. Dumat, Chem. Eur. J., 2020, 26, 14467–14473.
- Lipid particles functionalized with tailored fluorescent glycolipids to study Phagocytosis
In order to investigate the role of C-type lectin receptors in phagocytosis, we synthesize fluorescent glycolipids for the surface functionalization of lipid microparticles. The resulting fluorescent and targeted particles are used to visualize and study their phagocytic internalization induced by C-type lectin receptors.
Funding: ANR PhagoChemiForce (2020-2024, PI: F. Niedergang (Institut Cochin), partner: J-M Mallet)
Collaborations: Jacques Fattaccioli (PASTEUR, UMR8640), Florence Niedergang (Institut Cochin)
Publications:
B. Dumat, L. Montel, L. L. Pinon, P. Matton, L. Cattiaux, J. Fattaccioli and J.-M. M. Mallet, ACS Appl. Bio Mater., 2019, 2, 5118–5126.
- Past Projects
- Calcium-Rubies
In an earlier project, we have designed red-emitting calcium probes (called Calcium-Ruby) based on a chelating moiety (tetraacid BAPTA) and X-Rhodamine. Without calcium bound, the fluorescence of the X-rhodamine is quenched, but the complexed form is fluorescent during calcium spikes in neurons (in example X = H, Y = Cl: Kd = 30 µM – The Kd is adjusted by the substituents X or Y).
The lateral side chain with an azide function allows the immobilization on a quantum dot or on a modiflied dextan.

Gaillard, S.; Yakovlev, A.; Luccardini, C.; Oheim, M.; Feltz, A.; Mallet, J. Org. Lett. 2007, 9 (14), 2629–2632.
Collot, M.; Loukou, C.; Yakovlev, A. V.; Wilms, C. D.; Li, D.; Evrard, A.; Zamaleeva, A.; Bourdieu, L.; Léger, J. F.; Ropert, N.; Eilers, J.; Oheim, M.; Feltz, A.; Mallet, J. M. J. Am. Chem. Soc. 2012, 134 (36), 14923–14931.
Oheim, M.; van ’t Hoff, M.; Feltz, A.; Zamaleeva, A.; Mallet, J.-M.; Collot, M. Biochim. Biophys. Acta – Mol. Cell Res. 2014, 1843 (10), 2284–2306.
Collot, M.; Wilms, C. D.; Bentkhayet, A.; Marcaggi, P.; Couchman, K.; Charpak, S.; Dieudonné, S.; Häusser, M.; Feltz, A.; Mallet, J.-M. Elife 2015, 4, 1–18.
-
- H-Rubies (pH probes)
In a similar fashion, we have also designed pH probes based on the association of X-Rhodamine with pH-sensitive moities.

Despras, G.; Zamaleeva, A. I.; Dardevet, L.; Tisseyre, C.; Magalhaes, J. G.; Garner, C.; De Waard, M.; Amigorena, S.; Feltz, A.; Mallet, J.-M.; Collot, M. Chem. Sci. 2015, 6 (10), 5928–5937.
Zamaleeva, A. I.; Despras, G.; Luccardini, C.; Collot, M.; De Waard, M.; Oheim, M.; Mallet, J. M.; Feltz, A. Sensors (Switzerland) 2015, 15 (9), 24662–24680.
