A robust ultra-microporous cationic aluminum-based metal-organic framework with a flexible tetra-carboxylate linker
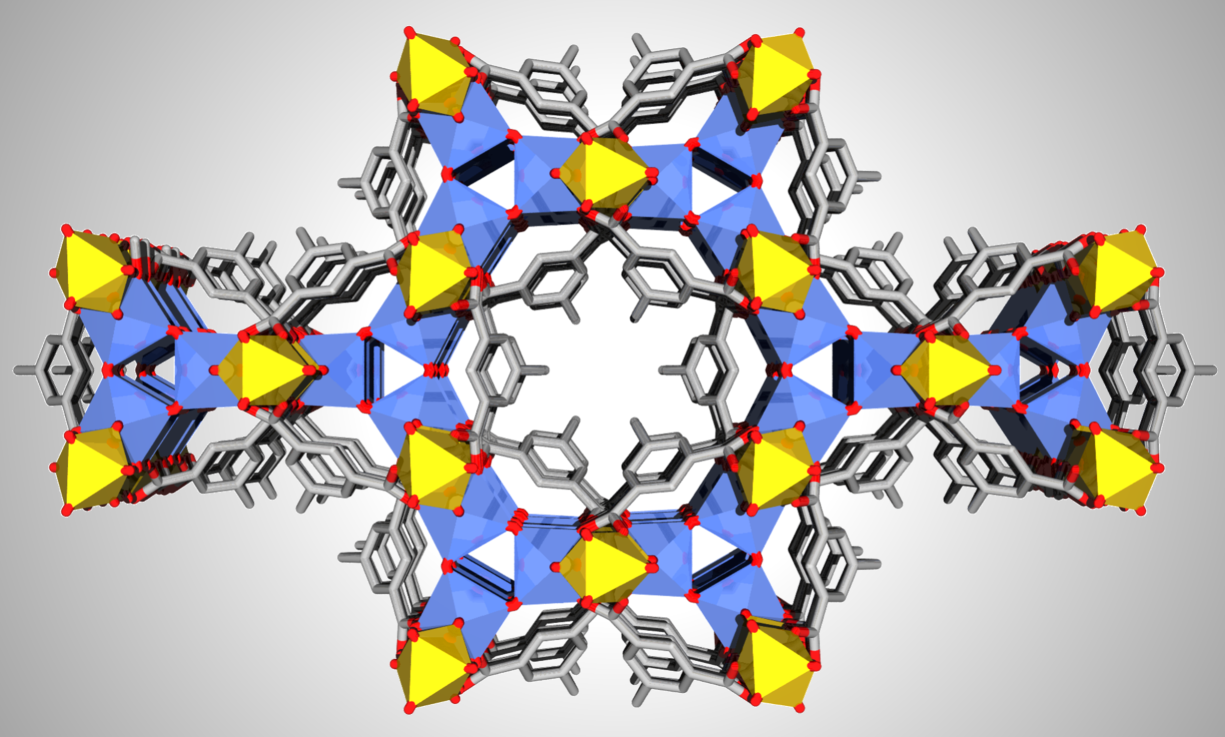
Al-based cationic metal-organic frameworks (MOFs) are uncommon. Here, we report a cationic Al-MOF, MIP-213(Al) ([Al18(μ2-OH)24(OH2)12(mdip)6]6Cl·6H2O) constructed from flexible tetra-carboxylate ligand (5,5′-Methylenediisophthalic acid; H4mdip). Its crystal structure was determined by the combination of three-dimensional electron diffraction (3DED) and high-resolution powder X-ray diffraction. The structure is built from infinite corner-sharing chains of AlO4(OH)2 and AlO2(OH)3(H2O) octahedra forming an 18-membered rings honeycomb lattice, similar to that of MIL-96(Al), a scarce Al-polycarboxylate defective MOF. Despite sharing these structural similarities, MIP-213(Al), unlike MIL-96(Al), lacks the isolated μ3-oxo-bridged Al-clusters. This leads to an ordered defective cationic framework whose charge is balanced by Cl- sandwiched between two Al-trimers at the corner of the honeycomb, showing strong interaction with terminal H2O coordinated to the Al-trimers. The overall structure is endowed by a narrow quasi-1D channel of dimension ~4.7 Å. The Cl- in the framework restrains the accessibility of the channels, while the MOF selectively adsorbs CO2 over N2 and possesses high hydrolytic stability.









 Realizing molecular “Designer Solids” by programmed assembly of building units taken form libraries is a very appealing objective. Recently, metal-organic frameworks (MOFs) have attracted a huge interest in this context. Here, we will focus on MOF-based electrochemical, photoelectron-chemical, photovoltaic, and sensor devices. Internal interfaces in MOF heterostructures are also of interest with regard to photon-upconversion and the fabrication of diodes.
Realizing molecular “Designer Solids” by programmed assembly of building units taken form libraries is a very appealing objective. Recently, metal-organic frameworks (MOFs) have attracted a huge interest in this context. Here, we will focus on MOF-based electrochemical, photoelectron-chemical, photovoltaic, and sensor devices. Internal interfaces in MOF heterostructures are also of interest with regard to photon-upconversion and the fabrication of diodes.
