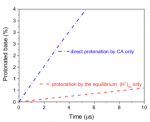
Carbonic acid H2CO3 (CA) is a key constituent of the universal CA/ bicarbonate/CO2 buffer maintaining the pH of both blood and the oceans. Here we demonstrate the ability of intact CA to quantita- tively protonate bases with biologically-relevant pKas and argue that CA has a previously unappreciated function as a major source of protons in blood plasma. We determine with high precision the temperature dependence of pKa(CA), pKa(T) = −373.604 + 16,500/T +56.478 ln T. At physiological-like conditions pKa(CA) = 3.45 (I =0.15 M, 37 °C), making CA stronger than lactic acid. We further demonstrate experimentally that CA decomposition to H2O and CO2 does not impair its ability to act as an ordinary carboxylic acid and to efficiently protonate physiological-like bases. The conse- quences of this conclusion are far reaching for human physiol- ogy and marine biology. While CA is somewhat less reactive than (H+)aq, it is more than 1 order of magnitude more abundant than (H+)aq in the blood plasma and in the oceans. In particular, CA is about 70× more abundant than (H+)aq in the blood plasma, where we argue that its overall protonation efficiency is 10 to 20× greater than that of (H+)aq, often considered to be the major protonating agent there. CA should thus function as a major source for fast in vivo acid–base reactivity in the blood plasma, possibly pene- trating intact into membranes and significantly helping to com- pensate for (H+)aq’s kinetic deficiency in sustaining the large proton fluxes that are vital for metabolic processes and rapid enzymatic reactions.

References:
Intact carbonic acid is a viable protonating agent for biological bases
Daniel Aminov, Dina Pines, Philip M. Kiefer, Snehasis Daschakraborty, James T. Hynes, and Ehud Pines
PNAS vol. 116 | no. 42 | 20837–20843
DOI: 10.1073/pnas.1909498116

A series of seminar in bio-inorganic chemistry, presented by M. Fontecave (CdF), G. Gasser (Chimie Paris-Tech), Clotilde Policar (ENS) and Raphaël Rodriguez (Institut Curie).
Consult the PSL – BIC Program 2019 – Semester 2
Room E012, salle des éléments, département de chimie de l’ENS, 24 rue Lhomond, 16h30 / 4pm30 — This seminar is founded by PSL (ANR 10-IDEX-0001-02)
The PSL-BIC programme can be found on https://clone.chimie.ens.fr/agenda/
To add PSL-BIC seminars to your agenda :
https://calendar.google.com/calendar/ical/pslbicseminars%40gmail.com/public/basic.ics
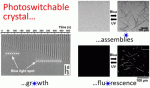
Self-assembled nucleobases, such as G-quartets or quadruplexes, have numerous applications, but light-responsive structures are limited to small, noncrystalline motifs. In addition, the assembly of the widely exploited azobenzene photochromic compounds can produce fluorescent crystals of extended dimensions but at the prize of sacrificing their photoswitchability. Here, we overcome inherent limitations of self-assembly with a new concept of supramolecular coassembly leading to materials with unprecedented properties. We show that the coassembly of guanosine monophosphate (GMP) with an azobenzene-containing DNA intercalator produces supramolecular crystals arranged through a combination of π–π, electrostatic, and hydrogen-bond interactions. The resulting crystals are 100 μm long, pH-sensitive, fluorescent, and can be photoreversibly disassembled/reassembled upon UV/blue irradiation. This allows us to perform operations such as dynamic photocontrol of a single-crystal growth, light-gated permeability in membrane-like materials, and photoswitchable fluorescence. We believe this concept critically expands the breadth of multifunctional materials attainable by self-assembly.

Press release available :
Quand une base et un intercalant d’ADN s’assemblent, il pousse des cristaux fluorescents photocommutables
References :
Photoswitchable Fluorescent Crystals Obtained by the Photoreversible Coassembly of a Nucleobase and an Azobenzene Intercalator
Li Zhou, Pascal Retailleau, Mathieu Morel, Sergii Rudiuk, and Damien Baigl
J. Am. Chem. Soc. 2019, 141, 9321−9329
DOI : 10.1021/jacs.9b02836

Liquid–liquid phase separation is thought to be a key organizing principle in eukaryotic cells to generate highly concentrated dynamic assemblies, such as the RNP granules. Numerous in vitro approaches have validated this model, yet a missing aspect is to take into consideration the complex molecular mixture and promiscuous interactions found in vivo. Here we report the versatile scaffold ArtiG to generate concentration-dependent RNA–protein condensates within living cells, as a bottom-up approach to study the impact of co-segregated endogenous components on phase separation. We demonstrate that intracellular RNA seeds the nucleation of the condensates, as it provides molecular cues to locally coordinate the formation of endogenous high-order RNP assemblies. Interestingly, the co-segregation of intracellular components ultimately impacts the size of the phase-separated condensates. Thus, RNA arises as an architectural element that can influence the composition and the morphological outcome of the condensate phases in an intracellular context.

Press release available :
Building artificial organelles to examine the formation of RNA-protein compartments
(in french : Utiliser des organelles artificielles pour examiner les transitions de phase contrôlant la formation des compartiments ARN-protéines)
References
RNA is a critical element for the sizing and the composition of phase-separated RNA-protein condensates
Marina Garcia-Jove Navarro, Shunnichi Kashida, Racha Chouaib, Sylvie Souquere, Gerard Pierron, Dominique Weil, Zoher Gueroui
Nature Communications
DOI : 10.1038/s41467-019-11241-6
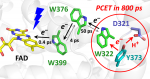
The animal-like cryptochrome of Chlamydomonas reinhardtii (CraCRY) is a recently discovered photoreceptor that controls the transcriptional profile and sexual life cycle of this alga by both blue and red light. CraCRY has the uncommon feature of efficient formation and longevity of the semireduced neutral form of its FAD cofactor upon blue light illumination. Tyrosine Y373 plays a crucial role by elongating, as fourth member, the electron transfer (ET) chain found in most other cryptochromes and DNA photolyases, which comprises a conserved tryptophan triad. Here, we report the full mechanism of light-induced FADH• formation in CraCRY using transient absorption spectroscopy from hundreds of femtoseconds to seconds. Electron transfer starts from ultrafast reduction of excited FAD to FAD•– by the proximal tryptophan (0.4 ps) and is followed by delocalized migration of the produced WH•+ radical along the tryptophan triad (∼4 and ∼50 ps). Oxidation of Y373 by coupled ET to WH•+ and deprotonation then proceeds in ∼800 ps, without any significant kinetic isotope effect, nor a pH effect between pH 6.5 and 9.0. The FAD•–/Y373• pair is formed with high quantum yield (∼60%); its intrinsic decay by recombination is slow (∼50 ms), favoring reduction of Y373• by extrinsic agents and protonation of FAD•– to form the long-lived, red-light absorbing FADH• species. Possible mechanisms of tyrosine oxidation by ultrafast proton-coupled ET in CraCRY, a process about 40 times faster than the archetypal tyrosine-Z oxidation in photosystem II, are discussed in detail.

Reference:
Ultrafast Oxidation of a Tyrosine by Proton-Coupled Electron Transfer Promotes Light Activation of an Animal-like Cryptochrome
Fabien Lacombat, Agathe Espagne, Nadia Dozova, Pascal Plaza,* Pavel Müller,* Klaus Brettel, Sophie Franz-Badur, Lars-Oliver Essen*
J. Am. Chem. Soc., 2019, 141, 13394-13409.
DOI: 10.1021/jacs.9b03680
Herein, we present the design of a comprehensive, physiologically relevant, easy-to-use and low-cost microfluidic and microscopic setup for the monitoring of Physcomitrella patens (P. patens) growth and development on a long-term basis. The experimental solution we developed is made of two parts (i) a microfluidic chip composed of a single layer of about a hundred flow-through microfluidic traps for the immobilization of protoplasts, and (ii) a low-cost, light-controlled, custom-made microscope allowing the continuous recording of the moss development in physiological conditions. We validated the experimental setup with three proofs of concepts: (i) the kinetic monitoring of first division steps and cell wall regeneration, (ii) the influence of the photoperiod on growth of the protonemata, and (iii) finally the induction of leafy buds using a phytohormone, cytokinin.

Press release available :
in french : Quand la microfluidique observe le développement cellulaire des plantes !
Reference :
K. Sakai, F. Charlot, T. Le Saux, S. Bonhomme, F. Nogué, J.C. Palauqui*, J. Fattaccioli*.
Design of a microfluidic and microscopic toolbox for the ultra-wide spatio-temporal study of plant protoplast development and physiology.
Plant Methods, 15 : 79 (2019) [doi]
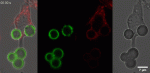
We designed a comprehensive, semi-automated and quantitative phagocytic assay, in conjunction with two types of microparticles showing monodisperse or polydisperse size distribution. We show that size is not a limiting factor of phagocytosis, but that phagocytosis depends on the total amount of surface area a cell is able to produce.
Phagocytosis by macrophages represents a fundamental process that consists in the uptake of pathogenic or cellular targets. The study of the role of the size of objects in their phagocytosis has lead to contradictory results in the last decades. To address this question, we designed a comprehensive, semi-automated and quantitative phagocytic assay, in conjunction with two types of microparticles showing mono disperse or polydisperse size distribution. We show that size is not a limiting factor of phagocytosis, but that phagocytosis depends on the total amount of surface area a cell is able to produce.

Reference :
A multiparametric and high-throughput assay to quantify the influence of target size on phagocytosis
L. Montel, L. Pinon, and J. Fattaccioli
Biophys. J. (2019)
https://doi.org/10.1016/j.bpj.2019.06.021

The Yaroslav Heyrovsky Prize in Molecular Electrochemistry was awarded to Christian Amatore, CNRS FRANCE Ecole Normale Superieure, Paris, France

The Jaroslav Heyrovsky Prize for Molecular Electrochemistry, supported by the International Society of Electrochemistry, may be awarded annually to a scientist who has made an important contribution to the field of molecular electrochemistry in the last 5 years.

Interactions between proteins play an essential role in metabolic and signaling pathways, cellular processes and organismal systems. We report the development of splitFAST, a fluorescence complementation system for the visualization of transient protein-protein interactions in living cells. Engineered from the fluorogenic reporter FAST (Fluorescence-Activating and absorption-Shifting Tag), which specifically and reversibly binds fluorogenic hydroxybenzylidene rhodanine (HBR) analogs, splitFAST displays rapid and reversible complementation, allowing the real-time visualization of both the formation and the dissociation of a protein assembly.

Press release available :
Spying on protein-protein interactions with fluorescent chemical-genetic hybrids
(in french : Espionner les interactions protéine-protéine avec des hybrides chemogénétiques fluorescents)
References:
A split fluorescent reporter with rapid and reversible complementation
Alison G. Tebo and Arnaud Gautier*
PASTEUR, Département de chimie, École normale supérieure, PSL University, Sorbonne Université́, CNRS, 24 Rue Lhomond, 75005 Paris, France
Nature Communications 10, 2822 (2019)
DOI : 10.1038/s41467-019-10855-0
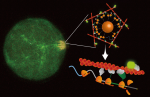
The name of messenger RNAs-“messenger », has always implicated the role of this class of RNA molecules as to transmit DNA information to protein, an intermediate. But now our data suggest that an mRNA can function more. Using a bottom-up approach to combine magnetic nanoparticles, synthetic RNAs, and human cell extract, all confined in water-in-oil droplets, we observed that a messenger RNA not only can be translated into functional proteins, but can also serve as a spatiotemporal scaffold, to concentrate the nascent proteins and their function at the translation site, and for the time being of translation. This temporary site-specific concentration is observed to trigger structural polymorphism of actin filament meshworks, the choreographers of cell shape and function. From a biological perspective, this phenomenon may represent a general mechanism of spatial control that cells use to produce functional diversity.

References:
Nanoparticle-based local translation reveals mRNA as a translation-coupled scaffold with anchoring function
Shunnichi Kashida, Dan Ohtan Wang, Hirohide Saito, and Zoher Gueroui
PNAS 2019
https://doi.org/10.1073/pnas.1900310116
















