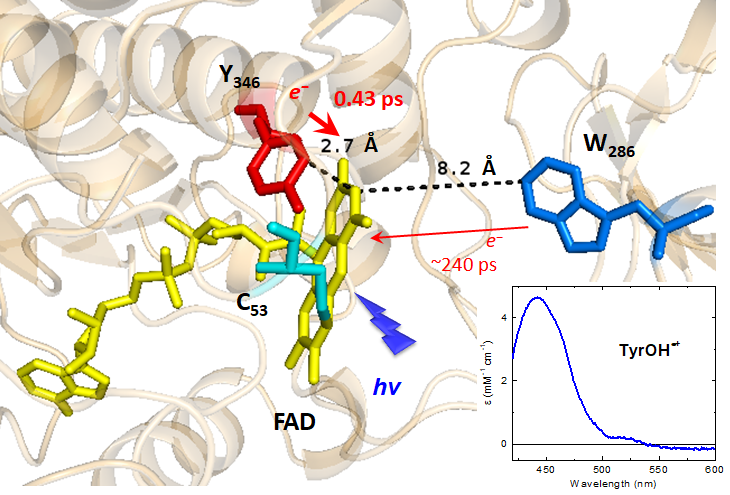
Flavoproteins often stabilize their flavin coenzyme by stacking interactions involving the isoalloxazine moiety of the flavin and an aromatic residue from the apoprotein. The bacterial FAD and folate-dependent tRNA methyltransferase TrmFO has the unique property of stabilizing its FAD coenzyme by an unusual H-bond-assisted π–π stacking interaction, involving a conserved tyrosine (Y346 in Bacillus subtilis TrmFO, BsTrmFO), the isoalloxazine of FAD and the backbone of a catalytic cysteine (C53). Here, the interaction between FAD and Y346has been investigated by measuring the photoinduced flavin dynamics of BsTrmFO in the wild-type (WT) protein, C53A and several Y346 mutants by ultrafast transient absorption spectroscopy. In C53A, the excited FAD very rapidly (0.43 ps) abstracts an electron from Y346, yielding the FAD˙−/Y346OH˙+ radical pair, while relaxation of the local environment (1.3 ps) of the excited flavin produces a slight Stokes shift of its stimulated emission band. The radical pair then decays via charge recombination, mostly in 3–4 ps, without any deprotonation of the Y346OH˙+ radical. Presumably, the H-bond between Y346 and the amide group of C53 increases the pKa of Y346OH˙+ and slows down its deprotonation. The dynamics of WT BsTrmFO shows additional slow decay components (43 and 700 ps), absent in the C53A mutant, assigned to excited FADox populations not undergoing fast photoreduction. Their presence is likely due to a more flexible structure of the WT protein, favored by the presence of C53. Interestingly, mutations of Y346 canceling its electron donating character lead to multiple slower quenching channels in the ps–ns regime. These channels are proposed to be due to electron abstraction either (i) from the adenine moiety of FAD, a distribution of the isoalloxazine–adenine distance in the absence of Y346 explaining the multiexponential decay, or (ii) from the W286 residue, possibly accounting for one of the decays. This work supports the idea that H-bond-assisted π–π stacking controls TrmFO’s active site dynamics, required for competent orientation of the reactive centers during catalysis.

Reference:
Ultrafast photoinduced flavin dynamics in the unusual active site of the tRNA methyltransferase TrmFO
Nadia Dozova, Fabien Lacombat, Charles Bou-Nader, Djemel Hamdane* and Pascal Plaza*
Phys. Chem. Chem. Phys., 2019, 21, 8743-8756
DOI: 10.1039/C8CP06072J
Ultrafast photoinduced flavin dynamics in the unusual active site of the tRNA methyltransferase TrmFO
