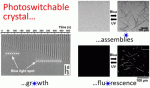
Self-assembled nucleobases, such as G-quartets or quadruplexes, have numerous applications, but light-responsive structures are limited to small, noncrystalline motifs. In addition, the assembly of the widely exploited azobenzene photochromic compounds can produce fluorescent crystals of extended dimensions but at the prize of sacrificing their photoswitchability. Here, we overcome inherent limitations of self-assembly with a new concept of supramolecular coassembly leading to materials with unprecedented properties. We show that the coassembly of guanosine monophosphate (GMP) with an azobenzene-containing DNA intercalator produces supramolecular crystals arranged through a combination of π–π, electrostatic, and hydrogen-bond interactions. The resulting crystals are 100 μm long, pH-sensitive, fluorescent, and can be photoreversibly disassembled/reassembled upon UV/blue irradiation. This allows us to perform operations such as dynamic photocontrol of a single-crystal growth, light-gated permeability in membrane-like materials, and photoswitchable fluorescence. We believe this concept critically expands the breadth of multifunctional materials attainable by self-assembly.

Press release available :
Quand une base et un intercalant d’ADN s’assemblent, il pousse des cristaux fluorescents photocommutables
References :
Photoswitchable Fluorescent Crystals Obtained by the Photoreversible Coassembly of a Nucleobase and an Azobenzene Intercalator
Li Zhou, Pascal Retailleau, Mathieu Morel, Sergii Rudiuk, and Damien Baigl
J. Am. Chem. Soc. 2019, 141, 9321−9329
DOI : 10.1021/jacs.9b02836
