Members involved:
Zoher GUEROUI, Christophe TRIBET
Being able to design genetically-encoded functional assemblies or synthetic organelles in living cells could provide numerous perspectives in biotechnology, material science, and nanomedicine. Our goal is to engineer proteins-, ribonucleoproteins, and lipid-based compartments in living cells with well-defined composition, structures, and properties. This enable to examine broad applications, including the implementation of novel functionalities to living cells or the production of well-defined colloids (lipidic compartments, magnetic particles, or membrane-less compartments) using cells as living factories. Although oriented by fundamental questions and biological applications, the use of living cell factories to produce remotely-controlled functional assemblies is of interest to design new eco-friendly sustainable processes.
– Engineering artificial RNA-protein organelles in cells
We recently developed a novel methodology for inducing the assembly of artificial RNA-protein organelles in human cells with specific biophysical and biochemical properties (ArtiGranules). This approach is used to examine how phase transitions, that are ubiquitous in non-living matter, allow biomolecules to condense into liquid or gel-like phases in order to organize cellular biochemistry. Additionally, the design of such synthetic compartments is used to engineer novel objects and functionalities in living cells.
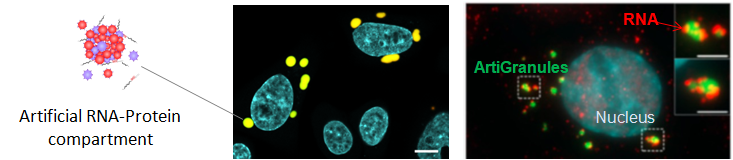
Engineering artificial RNA-Protein organelles in cells to study intracellular phase transitions and to produce novel functionalities.
Reference:
RNA is a critical element for the sizing and the composition of phase-separated RNA-protein condensates. Garcia-Jove Navarro M, Kashida K, Chouaib R, Souquere S, Pierron G, Weil D, and Gueroui Z. Nature Communications. 2019.
– Lipid-based capsules
In tight collaboration with membrane-protein biochemists, we propose to use bacterial strains « evolved » for lipid capsule preparation, which offers per se an original bio-based formulation of vesicles. These strains can express membrane proteins in high amounts. Specific modifications of a recombinant protein « scaffolds » enables to proliferate internal membranes, and form proteoliposomes densely covered by functional peptide sequences of interest (tags, elastin-like temperature-responsive moieties, etc.). We explore their labeling and loading (e.g. with enzymes and fluorescent proteins) and the genetic control on the shape of membranes (tubes, spheres, etc). Recent debates on extracellular vesicles that are secreted by various cell types, and loaded with enzymes, toxins, or DNA/RNA fragments, have raised questions on proteoliposome-based inter-cell communication. In this context, our approach offers a promising platform to the design of new therapeutic/diagnostic systems.
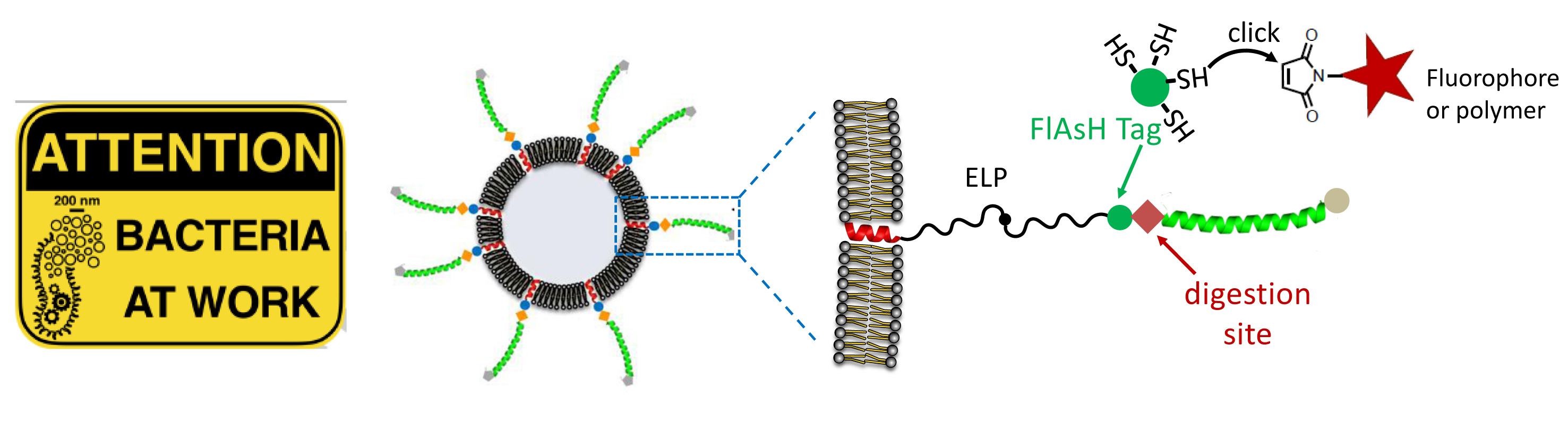
Bacteria-sourced proteoliposome to encode co-encapsulation, stimuli-responsive polymers, and click labeling.
