Actualités
Voir toutes les actualitésVoeux du Département
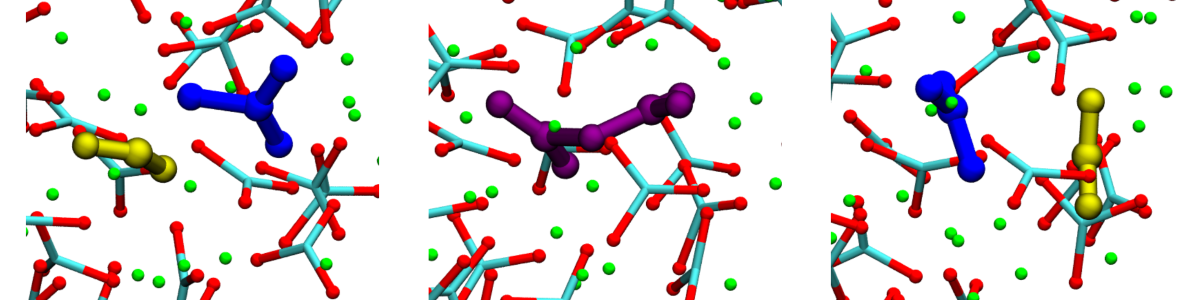
Signal strapping—a proteomic search technique for the discovery of metalloproteins
Journée Bram 2026 - 16 janvier
New article on photoaccumulation of long-lived electrons in a Ti-MOF
Winter Chemistry Day (for CPCV and IMAP members)
This year's Winter Chemistry Day (reserved to CPCV, IMAP and Chemistry Department members) is held on December 15th and for once, you will not get only chemistry presentations!
Seminar Pr. Gassensmith, 11am Dec. 4th
Jeremiah will give a talk on « Metal–Organic Frameworks as Dual-Function Platforms for Immunology and Therapeutics»
Recherche et formation
 |
 |
 |
 |
Suivez-nous
| Tweets by Chimie__ENS |