
Describing the dynamics of nuclei in molecules requires a potential energy surface, which is traditionally provided by the Born-Oppenheimer or adiabatic approximation. However, we also need to assign masses to the nuclei. There, the Born-Oppenheimer picture does not account for the inertia of the electrons, and only bare nuclear masses are considered. Nowadays, experimental accuracy challenges the theoretical predictions of rotational and vibrational spectra and requires the participation of electrons in the internal motion of the molecule. More than 80 years after the original work of Born and Oppenheimer, this issue has still not been solved, in general. Here, we present a theoretical and numerical framework to address this problem in a general and rigorous way. Starting from the exact factorization of the electron-nuclear wave function, we include electronic effects beyond the Born-Oppenheimer regime in a perturbative way via positiondependent corrections to the bare nuclear masses.

Consultez le communiqué associé à cet article : Corriger l’approximation de Born-Oppenheimer !
References:
On the Mass of Atoms in Molecules: Beyond the Born-Oppenheimer Approximation
Arne Scherrer, Federica Agostini, Daniel Sebastiani, E. K. U. Gross & Rodolphe Vuilleumier
PHYSICAL REVIEW X 25 août 2017
DOI: 10.1103/PhysRevX.7.031035

Anne BOUTIN a reçu, jeudi 31 août 2017, les insignes de Chevalier de la Légion d’Honneur par Gilberte CHAMBAUD, chimiste, Professeur émérite à l’Université Paris-Est Marne la Vallée.

Directrice du Département de Chimie de l’ENS, Anne BOUTIN est une spécialiste de la thermodynamique moléculaire. Elle développe et utilise des outils de simulation ainsi que des approches théoriques, pour étudier divers phénomènes touchant à la structure, la dynamique, la thermodynamique et la réactivité, de phases condensées, le plus souvent de fluides moléculaires confinés.


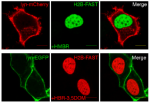
Yellow Fluorescence-Activating and absorption-Shifting Tag (Y-FAST, hereafter called FAST) is a 14-kDa protein tag giving a bright green-yellow fluorescent complex upon interaction with the fluorogenic dye 4-hydroxy-3-methylbenzylidene rhodanine (HMBR). Here, we report a collection of fluorogens enabling to tune the fluorescence color of FAST from greenyellow to orange and red. Beyond allowing multicolor imaging of FAST-tagged proteins in live cells, these fluorogens enable dynamic color switching because of FAST’s reversible labeling. This unprecedented behavior allows selective detection of FAST-tagged proteins in cells expressing both green and red fluorescent species through two-color crosscorrelation, opening exciting prospects to overcome spectral crowding and push the frontiers of multiplexed imaging.

Consultez le communiqué de presse associé à cet article : Observer les cellules avec un arc-en-ciel fluorescent !
References:
Dynamic multicolor protein labeling in living cells
Chenge Li, Marie-Aude Plamont, Hanna L. Sladitschek, Vanessa Rodrigues, Isabelle Aujard, Pierre Neveu, Thomas Le Saux, Ludovic Jullien and Arnaud Gautier
Chem. Sci., 2017, Advance Article
DOI: 10.1039/C7SC01364G
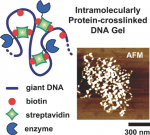
DNA micro- and nanogels—small-sized hydrogels made of a crosslinked DNA backbone—constitute new promising materials, but their functions have mainly been limited to those brought by DNA. Here a new way is described to prepare submicrometer-sized DNA gels of controllable crosslinking density that are able to embed novel functions, such as an enzymatic activity. It consists of using proteins, instead of traditional base-pairing assembly or covalent approaches, to form crosslinks inside individual DNA molecules, resulting in structures referred to as intramolecularly protein-crosslinked DNA gels (IPDGs). It is first shown that the addition of streptavidin to biotinylated T4DNA results in the successful formation of thermally stable IPDGs with a controllable crosslinking density, forming structures ranging from elongated to raspberry-shaped and pearl-necklace-like morphologies. Using reversible DNA condensation strategies, this paper shows that the gels can be reversibly actuated at a low crosslinking density, or further stabilized when they are highly crosslinked. Finally, by using streptavidin–protein conjugates, IPDGs with various enzymes are successfully functionalized. It is demonstrated that the enzymes keep their catalytic activity upon their incorporation into the gels, opening perspectives ranging from biotechnologies (e.g., enzyme manipulation) to nanomedicine (e.g., vectorization).

Consultez le communiqué de presse associé à cet article : Des microgels hybrides ADN-protéine
References:
Intramolecularly Protein-Crosslinked DNA Gels: New Biohybrid Nanomaterials with Controllable Size and Catalytic Activity
L. Zhou, M. Morel, S. Rudiuk and D. Baigl
Small 2017, 1700706
DOI: 10.1002/smll.201700706

Strategies to harness photosynthesis from living organisms to generate electrical power have long been considered, yet efficiency remains low. Here, we aimed to reroute photosynthetic electron flow in photosynthetic organisms without compromising their phototrophic properties. We show that 2,6-dimethyl-p-benzoquinone (DMBQ) can be used as an electron mediator to assess the efficiency of mutations designed to engineer a novel electron donation pathway downstream of the primary electron acceptor QA of Photosystem (PS) II in the green alga Chlamydomonas reinhardtii. Through the use of structural prediction studies and a screen of site-directed PSII mutants we show that modifying the environment of the QA site increases the reduction rate of DMBQ. Truncating the C-terminus of the PsbT subunit protruding in the stroma provides evidence that shortening the distance between QA and DMBQ leads to sustained electron transfer to DMBQ, as confirmed by chronoamperometry, consistent with a bypass of the natural QA°- to QB pathway.

Consultez le communiqué de presse associé à cet article : Des algues qui produisent de l’électricité !
References:
Redesigning the QA Binding Site of Photosystem II Allows Reduction of Exogenous Quinones
Han-Yi Fu, Daniel Picot, Yves Choquet, Guillaume Longatte, Adnan Sayegh, Jérôme Delacotte, Manon Guille-Collignon, Frédéric Lemaître, Fabrice Rappaport, and Francis-André Wollman
Nat. Commun., 8, 15274
DOI: 10.1038/ncomms15274
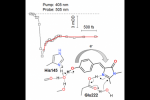
Because of growing applications in advanced fluorescence imaging, the mechanisms and dynamics of photoinduced reactions in reversibly photoswitchable fluorescent proteins are currently attracting much interest. We report the fi rst timeresolved study of the photoswitching of Dreiklang, so far the only fluorescent protein to undergo reversible photoinduced chromophore hydration. Using broadband femtosecond transient absorption spectroscopy, we show that the reaction is triggered by an ultrafast deprotonation of the chromophore phenol group in the excited state in 100 fs. This primary step is accompanied by coherent oscillations that we assign to its coupling with a low-frequency mode, possibly a deformation of the chromophore hydrogen bond network. A ground-state intermediate is formed in the picosecond− nanosecond regime that we tentatively assign to the deprotonated water adduct. We suggest that proton ejection from the phenol group leads to a charge transfer from the phenol to the imidazolinone ring, which triggers imidazolinone protonation by nearby Glu222 and catalyzes the addition of the water molecule.

Consultez le communiqué de presse associé à cet article : Comprendre le comportement d’un chromophore fluorescent !
References:
Photoinduced Chromophore Hydration in the Fluorescent Protein Dreiklang Is Triggered by Ultrafast Excited-State Proton Transfer Coupled to a Low-Frequency Vibration
Fabien Lacombat, Pascal Plaza, Marie-Aude Plamont, and Agathe Espagne
J. Phys. Chem. Lett. 2017, 8, 1489−1495
DOI: 10.1021/acs.jpclett.7b00348

Electrochemistry and confocal fluorescence microscopy were successfully combined to selectively bleach and monitor the fluorescence of NBD (7-nitrobenz-2-oxa-1,3- diazole)-labeled phospholipids of giant liposomes. Three types of giant unilamellar vesicles have been investigated, the fluorescent phospholipids being localized either mainly on their outer-, inner-, or both inner/outer leaflets. We established that only the fluorescent lipids incorporated in the outer leaflet of the vesicles underwent electrochemical bleaching upon reduction. The relative fluorescence intensity decay was quantified all along the electrochemical extinction through an original fluorescence loss in electrobleaching (FLIE) assay. As expected, the reorganization of the fluorescent phospholipids followed diffusion-driven dynamics. This was also evidenced by comparison with fluorescence loss in photobleaching (FLIP) and the corresponding numerical model. The value of the lateral diffusion coefficient of phospholipids was found to be similar to that obtained by other methods reported in the literature. This versatile and selective bleaching procedure appears reliable to explore important biological and pharmacological issues.

Consultez le communiqué de presse associé à cet article : Vers l’imagerie de la dynamique d’une membrane cellulaire !
References:
Selective Electrochemical Bleaching of the Outer Leaflet of Fluorescently Labeled Giant Liposomes
Ana Isabel Perez Jimenez, Lylian Challier, Eric Aït-Yahiatène, Jérôme Delacotte, Eric Labbé, and Olivier Buriez
Chem. Eur. J., 23,1–8, 2017
DOI: 10.1002/chem.201605786

Pour s’immerger dans cette longue et mystérieuse histoire, La Méthode scientifique de France Culture a le plaisir d’accueillir Bertrand Guillot, physico-chimiste, directeur de recherche au CNRS, rattaché au laboratoire de physique théorique de la matière condensée de l’UPMC, Nicolas Lévy, professeur agrégé de chimie au département chimie de l’ENS, rattaché au pôle de physico-chimie théorique et François-Xavier Coudert, chargé de recherche au CNRS, rattaché à l’Institut de Recherche de Chimie Paris.
L’eau, une histoire trouble : c’est le problème qui va occuper La Méthode scientifique dans l’heure qui vient. A savourer en Podcast !


Chaque année, les éditeurs du Journal of Chemical Physics mettent en avant la publication des articles les plus innovants et les plus influents en physico-chimie. Dans le 2017 Editor’s Choice, les éditeurs ont sélectionné 73 des nombreux articles remarquables du JCP de 2017 qui présentent des recherches significatives dans des domaines expérimentaux et théoriques. Ces articles sont disponibles gratuitement en ligne jusqu’à la fin de 2018.

L’article « Efficient molecular density functional theory using generalized spherical harmonics expansions » fait parti des 73 articles sélectionnées par le 2017 Editor’s Choice


Arnaud GAUTIER, Maître de Conférence au sein du Département de Chimie de l’École normale supérieure est lauréat du financement ERC Consolidator Grant 2016 et Médaille de Bronze du CNRS.
Visionnez son interview où il nous explique ce qu’est la chémobiologie et nous détaille ses projets à venir !
What is Chemical Biology?
Chemical biology gathers a community of scientists interested in science encompassing both chemistry and biology. Even though it originates from the accretion of several disciplines at the intersection of chemistry and biology (e.g. bioorganic chemistry, biochemistry, molecular biology and pharmacology), chemical biology now extends beyond these boundaries, reaching a point where some scientists define themselves as chemical biologists. The reasons why chemists and biologists find a common interest in chemical biology are complementary: on one hand, for chemists, the complexity of biological systems appears like the ultimate playground in terms of chemical reactivity and analytical challenges; on the other hand, for biologists, chemistry appears like the most appropriate level of description to unravel biological processes. This led to the creation of a dynamic scientific community interested in understanding and manipulating biological systems in new ways using the knowledge of chemistry. Chemical biology enables in particular the conception and discovery of powerful molecules to observe and virtually manipulate any cellular process, allowing for addressing biological problems that would otherwise remain elusive.
What is an ERC Consolidator Grant?
The european research council (ERC) is a European research agency, whose goal is to encourage the highest quality research in Europe and to support frontier research across all fields, on the basis of scientific excellence as sole criterion. One of the goal is to support the work of the next generation of research leaders in Europe.
The ERC Consolidator Grant aims at supporting young principal investigators to consolidate their independence. These grants are designed to back up researchers who want to establish their research teams and develop a successful career in Europe.
What is my project about ?
My project aims at pushing the frontiers of biological imaging. Today, biological imaging is an essential tool to understand living systems, which are complex systems driven by a set of dynamic biological events that are tightly orchestrated from the single molecule level to the entire organism level. The project FLUOSWITCH aims at providing chemical biology tools to address current challenges in biological imaging, such as: (1) How to image a large number of biomolecules in single cells to address the full complexity of a biological system? (2) How to increase the spatio-temporal resolution of biomolecular imaging to better understand biological processes both in space and time? (3) How to image endogenous biomolecules in their native environnement with high sensitivity and minimal perturbation of the studied system? (4) How to map active cell circuits in a whole organism?






















