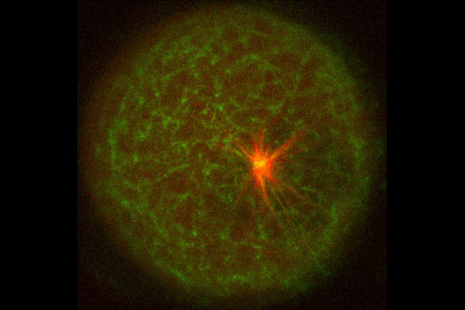
Coordination between actin filaments and microtubules is critical to complete important steps during cell division. For instance, cytoplasmic actin filament dynamics play an active role in the off-center positioning of the spindle during metaphase I in mouse oocytes or in gathering the chromosomes to ensure proper spindle formation in starfish oocytes, whereas cortical actin filaments control spindle rotation and positioning in adherent cells or in mouse oocytes. Several molecular effectors have been found to facilitate anchoring between the meiotic spindle and the cortical actin. In vitro reconstitutions have provided detailed insights in the biochemical and physical interactions between microtubules and actin filaments. Yet how actin meshwork architecture affects microtubule dynamics is still unclear. Here, we reconstituted microtubule aster in the presence of a meshwork of actin filaments using confined actin-intact Xenopus egg extracts. We found that actin filament branching reduces the lengths and growth rates of microtubules and con- strains the mobility of microtubule asters. By reconstituting the interaction between dynamic actin filaments and microtubules in a minimal system based on purified proteins, we found that the branching of actin filaments is sufficient to block microtubule growth and trigger microtubule disassembly. In a further exploration of Xenopus egg extracts, we found that dense and static branched actin meshwork perturbs monopolar spindle assembly by constraining the motion of the spindle pole. Interestingly, monopolar spindle assembly was not constrained in conditions supporting dynamic meshwork rearrangements. We propose that branched actin filament meshwork provides physical barriers that limit microtubule growth.

Pour plus d’informations, n’hésitez pas à consulter le communiqué de presse publié par l’Institut des Sciences Biologiques du CNRS : Actine et microtubules : régulations croisées
References:
Actin-Network Architecture Regulates Microtubule Dynamics
Colin A, Singaravelu P, Théry M, Blanchoin L, Gueroui Z.
Curr Biol. (16) 2018 : 2647-2656
doi: 10.1016/j.cub.2018.06.028
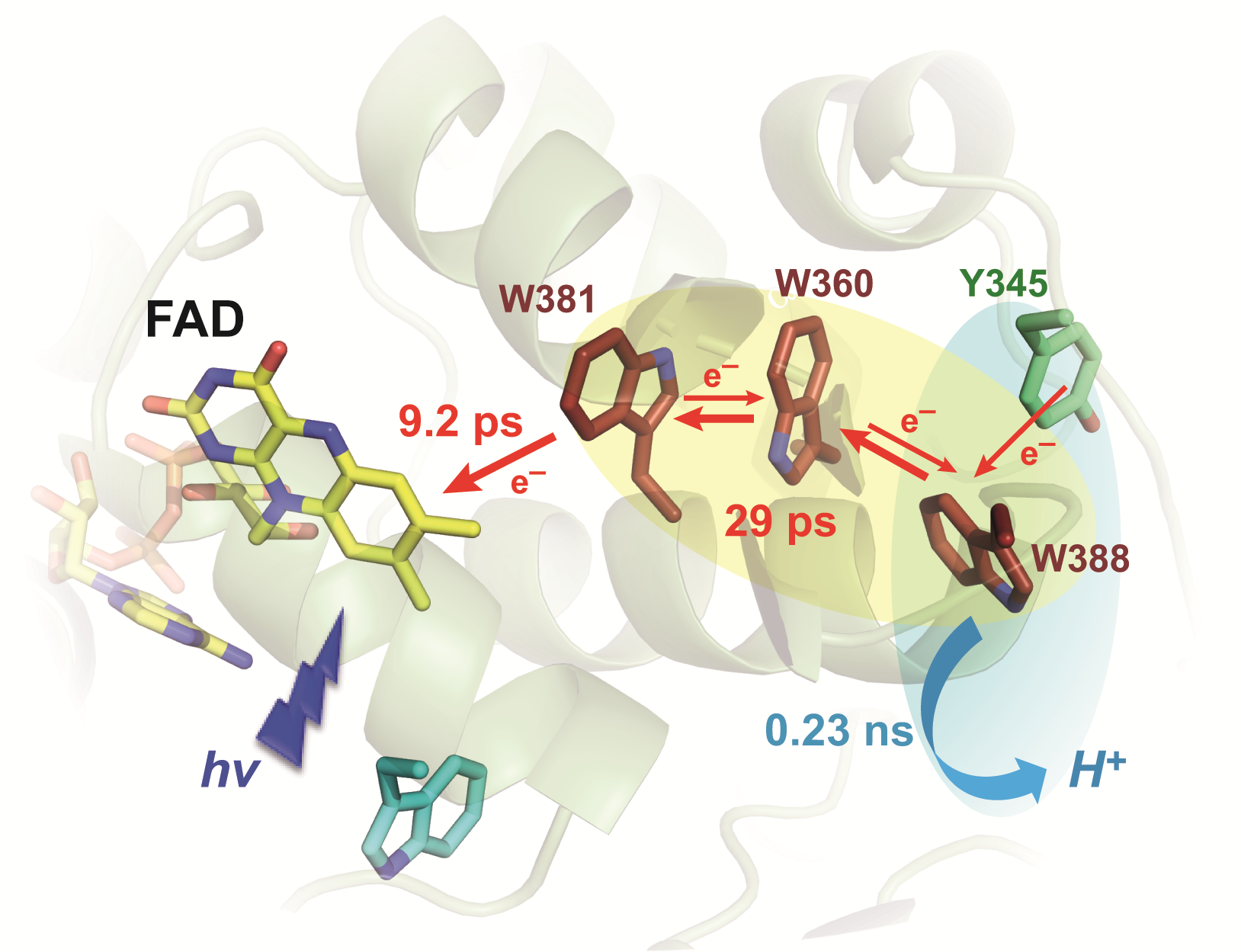
Class II photolyases utilize for the photoreduction of their flavin cofactor (FAD) a completely different tryptophan triad than most other photolyases and cryptochromes. To counter sped-up back electron transfer, they evolved an unusually fast deprotonation of the distal tryptophanyl radical cation (WH˙+) that is produced after excitation of the flavin. We studied the primary aspects of oxidized FAD photoreduction by ultrafast transient absorption spectroscopy, using the class II photolyase from Methanosarcina mazei. With a time constant of 9.2 ps, the initial reduction step of the excited flavin by the proximal W381 tryptophan proceeds almost twentyfold slower than in other photolyases carrying oxidized FAD, most likely because of the larger distance between the flavin and the proximal tryptophan. The thus formed W381H˙+ radical is tracked by transient anisotropy measurements to migrate in 29 ps with delocalization over several members of the tryptophan triad. This 29 ps phase also includes the decay of a small fraction of excited flavin, reacting on a slower timescale, and partial recombination of the FAD˙−/WH˙+ radical pair. A final kinetic phase in 230 ps is assigned to the deprotonation of W388H˙+ that occurs in competition with partial charge recombination. Interestingly, we show by comparison with the Y345F mutant that this last phase additionally involves oxidation of the Y345 phenolic group by W388H˙+, producing a small amount of neutral tyrosyl radical (YO˙). The rate of this electron transfer step is about six orders of magnitude faster than the corresponding oxidation of Y345 by the deprotonated W388˙ radical. Unlike conventional photolyases, where the electron hole accumulates on the distal tryptophan before the much slower tryptophanyl deprotonation, our data show that delocalized hole transport is concomitantly concluded by ultrafast deprotonation of W388H˙+.

References:
Delocalized hole transport coupled to sub-ns tryptophanyl deprotonation promotes photoreduction of class II photolyases
Fabien Lacombat, Agathe Espagne, Nadia Dozova, Pascal Plaza*, Elisabeth Ignatz, Stephan Kiontke and Lars-Oliver Essen
Phys. Chem. Chem. Phys., 2018, 20, 25446-25457
DOI: 10.1039/C8CP04548H
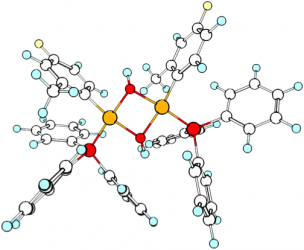
The mechanism of boron-to-nickel transmetalation, the key step of the nickel-catalyzed Suzuki-Miyaura (SM) coupling, was examined both experimentally and theoretically. Dinuclear μ -hydroxo-bridged complexes formed by reaction of trans[ArNi(PR3)2X] with hydroxide are not directly involved in transmetalation, but they rather act as a resting state for the catalyst. The base/boronic acid ratio is the crucial parameter, as it modulates the extent of formation of these dinuclear species and thus tunes the catalytic activity. These findings explain some limitations encountered in practical applications of nickel-catalyzed S-M couplings and suggest how to tailor the experimental conditions in order to overcome these difficulties.

Pour plus d’information, consultez le communiqué de presse associé à cet article : Rationaliser une catalyse au nickel entre théorie et expérience !
References:
Taming Nickel-Catalyzed Suzuki-Miyaura Coupling: A Mechanistic Focus on Boron-to-Nickel Transmetalation
Pierre-Adrien Payard, Luca Alessandro Perego, Ilaria Ciofini, and Laurence Grimaud*
ACS Catal. 2018, 8, 4812−4823
DOI: 10.1021/acscatal.8b00933

Arnaud GAUTIER, Maître de Conférence au Pôle Chimie Biophysique du Département de Chimie de l’ENS, a été nominé « membre junior » à l’Institut Universitaire de France (IUF).
L’IUF a pour mission de favoriser le développement de la recherche de haut niveau dans les universités et de renforcer l’interdisciplinarité. Créé par le décret du 26 août 1991, sous la forme d’un service du ministère de l’Enseignement supérieur et de la Recherche, il vient de fêter ses 20 ans.
Les enseignants-chercheurs qui y sont nommés sont distingués pour l’excellence de leur activité scientifique, attestée par leur rayonnement international.

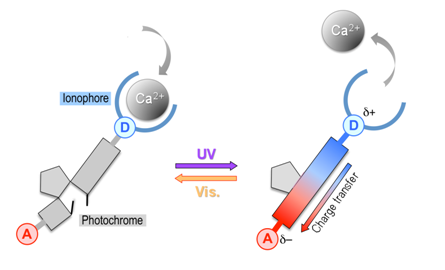
The synthesis and characterisation of a novel Reversibly Photoswitchable Chelator (RPC) of calcium ions, designed as a stepping stone towards producing pulses of calcium concentration in an aqueous environment, is reported. This RPC is constituted of a photochromic diarylethene core connected on one side to a BAPTA (1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid) calcium chelator and, on the other side, to an electron-withdrawing group. The operation principle consists in photoswitching on and off an intramolecular charge transfer between one nitrogen atom of the BAPTA moiety and the electron-withdrawing group, thereby modulating the chelating affinity of BAPTA for calcium ions. Solubility of the compound in a partially aqueous solvent was achieved by grafting a short PEG (polyethylene glycol) tail to the electron-withdrawing group. A reduction of the affinity for calcium ions upon photoswitching by a factor of 3–4, in the hundred nM range of dissociation constant, is reported and constitutes a proof of concept of this type of RPC.

References:
A photochromic calcium chelator is reported to reversibly reduce its affinity for calcium upon photoswitching in aqueous media.
Nadia Dozova,Guillaume Pousse, Bogdan Barnych, Jean-Maurice Mallet, Janine Cossy, Bernard Valeur, Pascal Plaza
J. Photochem., 2018, 360, 181
DOI:10.1016/j.jphotochem.2018.04.029

La Médaille Lavoisier, qui commémore l’œuvre d’Antoine-Laurent Lavoisier, est attribuée en reconnaissance de services éminents rendus aux sciences de la chimie.
Le 11 avril dernier, le Conseil d’administration a décidé à l’unanimité de décerner la Médaille Lavoisier au professeur Christian Amatore, directeur de recherche de classe exceptionnelle au CNRS (département de chimie de l’ENS), membre de l’Académie des sciences (où il est délégué à l’Éducation et à la Formation depuis 2011), pour l’ensemble de ses remarquables réalisations scientifiques, tout particulièrement pour ses travaux pionniers tant théoriques qu’expérimentaux en électrochimie moléculaire et analytique ayant conduit au développement des ultra-microélectrodes et de leurs applications.

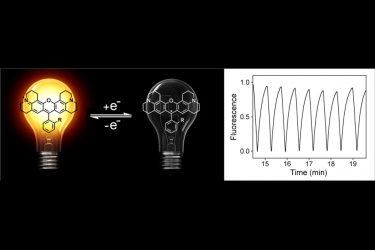
Three rhodamine derivatives exhibiting electrofluorochromic properties were investigated by cyclic voltammetry and UVeVis/fluorescence spectroelectrochemistry. Rhodamine 101 (Rh101, compound 1) was used as a reference model. In compound 2, the carboxylate anion of Rh101 was replaced by an alkyne moiety to allow further functionalization. The compound 3 was prepared from 2 by conversion of the alkyne to a triazole group bearing an alkyl chain with an alcohol function. These three rhodamine derivatives exhibited similar electrochemical behaviors. Their mono-electronic reductions produced the corresponding radical species which were stable on the time-scale of cyclic voltammetry. Additional reduction of electrogenerated radicals produced unstable anions which underwent subsequent chemical reaction, most likely protonation. Based on cyclic voltammetry investigations, absorption and fluorescence spectroelectrochemistry were then performed on compounds 1, 2, 3 and their parent reduced radicals 1a, 2a, 3a. UVeVis spectroelectrochemistry, combined with TD-DFT calculation, confirmed the formation of radicals upon mono-electronic reduction of starting rhodamines. Fluorescence spectroelectrochemistry showed that, contrary to their parent molecules, electrogenerated radicals were non-fluorescent. Electrochemical fluorescence extinction was successfully achieved with all studied compounds. Moreover, compound 1 underwent on/off switching between fluorescent and nonfluorescent states repeatedly. Also, recovery of fluorescence in compound 3 was observed, which open interesting opportunities for the development of versatile rhodamine-based probes.

Pour plus d’information,consultez le communiqué de presse associé à cet article :
L’électrofluorochromisme : quand l’électrochimie rencontre la fluorescence !
References:
Electrochemical switching fluorescence emission in rhodamine derivatives
Martina Cízkova, Laurent Cattiaux, Jean-Maurice Mallet, Eric Labbé, Olivier Buriez
Electrochimica Acta 260 (2018) 589-597
doi : 10.1016/j.electacta.2017.12.104

Christian AMATORE have been elected as Corresponding Member of the Brazilian Academy of Sciences.


High-purity cardiomyocytes (CMs) derived from human induced pluripotent stem cells (hiPSCs) are promising for drug development and myocardial regeneration. However, most hiPSC-derived CMs morphologically and functionally resemble immature rather than adult CMs, which could hamper their application. Here, we obtained high-quality cardiac tissue-like constructs (CTLCs) by cultivating hiPSC-CMs on low-thickness aligned nanofibers made of biodegradable poly(D,L-lactic-co-glycolic acid) polymer. We show that multilayered and elongated CMs could be organized at high density along aligned nanofibers in a simple one-step seeding process, resulting in upregulated cardiac biomarkers and enhanced cardiac functions. When used for drug assessment, CTLCs were much more robust than the 2D conventional control.We also demonstrated the potential of CTLCs for modeling engraftments in vitro and treating myocardial infarction in vivo. Thus, we established a handy framework for cardiac tissue engineering, which holds high potential for pharmaceutical and clinical applications.

Pour plus d’information, consultez le communiqué de presse associé à cet article :
Réparer le cœur après un infarctus à l’aide de cellules souches !
References:
Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium
Junjun Li, Itsunari Minami, Motoko Shiozaki, Leqian Yu, Shin Yajima, Shigeru Miyagawa, Yuji Shiba, Nobuhiro Morone, Satsuki Fukushima, Momoko Yoshioka, Sisi Li, Jing Qiao, Xin Li, Lin Wang, Hidetoshi Kotera, Norio Nakatsuji, Yoshiki Sawa, Yong Chen, and Li Liu
Stem Cell Reports, 2017, 9, 1546–1559
DOI : 10.1016/j.stemcr.2017.09.007
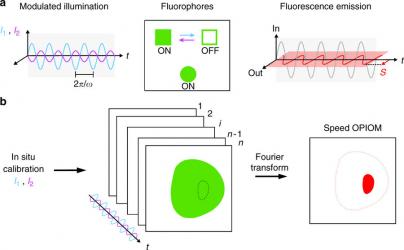
We present speed out-of-phase imaging after optical modulation (OPIOM), which exploits reversible photoswitchable fluorophores as fluorescent labels and combines optimized periodic illumination with phase-sensitive detection to specifically retrieve the label signal. Speed OPIOM can extract the fluorescence emission from a targeted label in the presence of spectrally interfering fluorophores and autofluorescence. Up to four fluorescent proteins exhibiting a similar green fluorescence have been distinguished in cells either sequentially or in parallel. Speed OPIOM is compatible with imaging biological processes in real time in live cells. Finally speed OPIOM is not limited to microscopy but is relevant for remote imaging as well, in particular, under ambient light. Thus, speed OPIOM has proved to enable fast and quantitative live microscopic and remote-multiplexed fluorescence imaging of biological samples while filtering out noise, interfering fluorophores, as well as ambient light.

Pour plus d’information, consultez le communiqué de presse associé à cet article :
Au-delà des frontières de la bioimagerie !
References:
Resonant out-of-phase fluorescence microscopy and remote imaging overcome spectral limitations
Jérôme Quérard, Ruikang Zhang, Zsolt Kelemen, Marie-Aude Plamont, Xiaojiang Xie, Raja Chouket, Insa Roemgens, Yulia Korepina, Samantha Albright, Eliane Ipendey, Michel Volovitch, Hanna L. Sladitschek, Pierre Neveu, Lionel Gissot, Arnaud Gautier, Jean-Denis Faure, Vincent Croquette, Thomas Le Saux & Ludovic Jullien
Nature Communications 8, 969 (2017)
DOI: 10.1038/s41467-017-00847-3



















