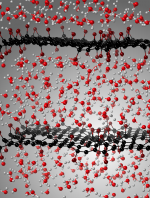
Graphene oxide is a rising star among 2D materials, yet its interaction with liquid water remains a fundamentally open question: experimental characterization at the atomic scale is difficult, and modeling by classical approaches cannot properly describe chemical reactivity. Here, we bridge the gap between simple computational models and complex experimental systems, by realistic first-principles molecular simulations of graphene oxide (GO) in liquid water. We construct chemically accurate GO models and study their behavior in water, showing that oxygen-bearing functional groups (hydroxyl and epoxides) are preferentially clustered on the graphene oxide layer. We demonstrated the specific properties of GO in water, an unusual combination of both hydrophilicity and fast water dynamics. Finally, we evidence that GO is chemically active in water, acquiring an average negative charge of the order of 10 mC m−2. The ab initio modeling highlights the uniqueness of GO structures for applications as innovative membranes for desalination and water purification.
Press Release (in french) by INC CNRS : Un nouveau modèle pour les interactions entre l’oxyde de graphène et l’eau
References:
Structure and chemistry of graphene oxide in liquid water from first principles
Félix Mouhat, François-Xavier Coudert & Marie-Laure Bocquet
Nature Communications volume 11, Article number: 1566 (2020)
doi: 10.1038/s41467-020-15381-y
