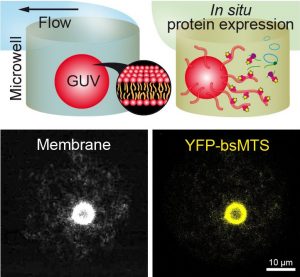
Giant unilamellar vesicles (GUVs) are an ideal model to study cellular membrane functions in vitro, yet difficult to manipulate due to their fragile nature, especially when subjected to dynamic change of their external microenvironment. Here, an original microfluidic concept is introduced for constraint-free confinement of individual GUVs in microchambers with a dynamically exchangeable outer medium. GUVs self-confine in an array of laterally separated microchambers by sedimentation, avoiding any mechanical constraint while allowing diffusion-based exchange of the outer medium via a microfluidic channel and time-resolved microscopy observation. The geometric and flow parameters optimizing medium exchange while preventing GUV from lifting out are numerically established. Different aqueous solutions separated by air plugs can be flowed into the channel by taking advantage of a polydimethylsiloxane-based hydrophilic channel wall. The possibility to manipulate microliter sample volumes for in situ observation of protein cell-free expression is also exploited. It is found that the membrane-targeting sequence of Bacillus subtilis MinD binds to GUVs and induces extensive membrane tubulation. This technically simple method offers a robust way to confine GUVs and dynamically control their outer medium, thus constituting an ideal platform to study the spatio-temporal response of reconstituted membranes and/or synthetic cell studies subjected to dynamic micro-environments.
Published in Advanced Materials Technologies: https://doi.org/10.1002/admt.202301976
by Syed Kaabir Ali, Catherine Sella, Vadim Dilhas, Barbara Jacková, Marina Mariconti, Clara Gomez-Cruz, Laurent Thouin, Mathieu Morel, Damien Baigl, and Ayako Yamada*
by Syed Kaabir Ali, Catherine Sella, Vadim Dilhas, Barbara Jacková, Marina Mariconti, Clara Gomez-Cruz, Laurent Thouin, Mathieu Morel, Damien Baigl, and Ayako Yamada*