Fluorescence imaging is widely used in the life sciences to visualize and analyze biological structures and processes. One of the main limitations is the photobleaching of fluorophores, i.e. their gradual photodegradation by the light used to excite fluorescence. Fluorophore photobleaching limits the duration and time resolution of experiments and degrades image quality. It also generates reactive oxygen species (ROS), which are toxic to living samples. Currently, two main approaches are used to control photobleaching. The first is to reduce the dose of excitation light, but this is usually at the expense of signal-to-noise ratio and/or time resolution. The second approach is to modify the fluorophore environment, generally to make it more reducing (removal of oxygen, addition of anti-oxidants) in order to neutralize ROS. However, this second approach is only of limited effectiveness, and it can disrupt the physiology of the biological systems of interest.
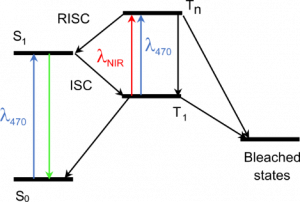
Principle of our method. The triplet excited state T1, a precursor of bleached states, is formed under visible excitation of the fluorophore from the bright singlet excited state S1. Near-infrared co-illumination promotes T1 into the higher triplet state Tn, from which the fluorophore returns to S1 by RISC.
In a paper published this month in Nature Biotechnology (https://doi.org/10.1038/s41587-023-01893-7), we describe a new method to reduce the photobleaching of fluorescent proteins and the associated phototoxicity. Our method exploits a photophysical process known as reverse intersystem crossing (RISC) to depopulate the triplet excited state of fluorophores, a precursor of photobleaching and a source of ROS. We induce RISC by co-illuminating fluorescent proteins at a near-infrared (NIR) wavelength absorbed by their triplet excited state, during their excitation with visible light. In our paper, we successfully apply this dual illumination method to wide-field fluorescence imaging of live eukaryotic and prokaryotic cells labelled with a wide range of green and yellow fluorescent proteins. We achieve a typical reduction in photobleaching by a factor of ~4, greater than that obtained with conventional antifading media. We show that our method can, for example, substantially improve the tracking of fluorescently-labelled replisomes in live E. coli cells. Finally, we show that it also reduces phototoxicity due to fluorescent protein excitation in growing bacteria and primary mouse neutrophils.
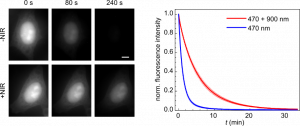
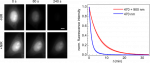
NIR co-illumination reduces the photobleaching of EGFP-labelled HeLa cells. Left: Fluorescence images of fixed HeLa cells labelled with EGFP and continuously illuminated at 470 nm alone (top) or combined with 900 nm (bottom). I470 = 32 W/cm2. I900 = 2 kW/cm2. Scale bar, 10 μm. Right: Photobleaching kinetics of HeLa cells. Values are mean ± s.d. (n = 12 cells).
Our method is efficient, technically easy to implement and compatible with living samples. It should therefore be widely adopted and provide access to a variety of new biological information, by allowing the use of higher light doses without adverse effects. Our work is also pioneering the use of RISC in fluorescence imaging, and can be expected to spark more applications in the near future, for example to other fluorophores or other imaging techniques. It should therefore have a major impact in the field.
Contacts: Agathe Espagne (agathe.espagne@ens.psl.eu) and Thomas Le Saux (thomas.lesaux@ens.psl.eu)
This work was carried out in collaboration with Lydia Robert (Micalis Institute, INRAE, Jouy-en-Josas, France).
Reference of the publication:
L. Ludvikova, E. Simon, M. Deygas, T. Panier, M.-A. Plamont, J. Ollion, A. Tebo, M. Piel, L. Jullien, L. Robert*, T. Le Saux* & A. Espagne*. Near-infrared co-illumination of fluorescent proteins reduces photobleaching and phototoxicity. Nat. Biotechnol. (2023). https://doi.org/10.1038/s41587-023-01893-7
