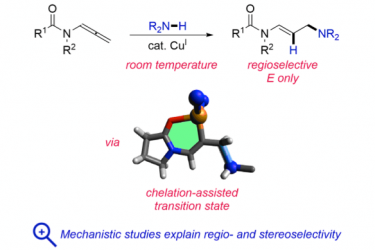
Experimental and theoretical mechanistic studies on the Cu(OTf)2-catalyzed hydroamination reaction of terminal allenes with secondary amines reveal that in-situ generated cationic Cu(I) is the catalytically active species and explain the observed regio- and stereoselectivity for the unbranched E product. Insight about the structure of the relevant transition states allowed the generalization of this methodology to allenamides and N-allenylcarbamates under unprecedentedly mild and functional group tolerant conditions. Chelation effect by the amide oxygen in addition to electronic effects explain the high innate reactivity of this class of substrates.

Pour plus d’information, consultez le communiqué associé à cet article :
Quand théorie et expérience dissèquent une catalyse au cuivre !
References:
Copper-Catalyzed Hydroamination of Allenes: from Mechanistic Understanding to Methodology Development
Luca Alessandro Perego, Rémi Blieck, Antoine Groué, Florian Monnier, Marc Taillefer, Ilaria Ciofini*, Laurence Grimaud*
ACS Catalysis, 2017, 7 (7), pp 4253–4264
DOI: 10.1021/acscatal.7b00911
Copper-Catalyzed Hydroamination of Allenes: from Mechanistic Understanding to Methodology Development
