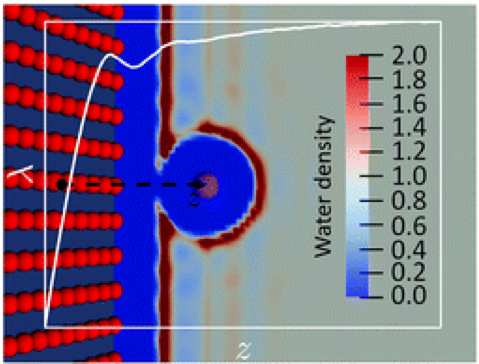
Beyond the dielectric continuum description initiated by Marcus theory, the standard theoretical approach to study electron transfer (ET) reactions in solution or at interfaces is to use classical force field or ab initio molecular dynamics simulations. We present here an alternative method based on liquid-state theory, namely molecular density functional theory, which is numerically much more efficient than simulations while still retaining the molecular nature of the solvent. We begin by reformulating molecular ET theory in a density functional language and show how to compute the various observables characterizing ET reactions from an ensemble of density functional minimizations. In particular, we define within that formulation the relevant order parameter of the reaction, the so-called vertical energy gap, and determine the Marcus free energy curves of both reactant and product states along that coordinate. Important thermodynamic quantities such as the reaction free energy and the reorganization free energies follow. We assess the validity of the method by studying the model Cl0 / Cl+ and Cl0 / Cl- ET reactions in bulk water for which molecular dynamics results are available. The anionic case is found to violate the standard Marcus theory. Finally, we take advantage of the computational efficiency of the method to study the influence of a solid–solvent interface on the ET, by investigating the evolution of the reorganization free energy of the Cl0 / Cl+ reaction when the atom approaches an atomistically resolved wall.

N’hésitez pas à consulter le communiqué de presse associé à cet article : Quand la théorie de Marcus se confronte à la DFT !
References :
A molecular density functional theory approach to electron transfer reactions
Guillaume Jeanmairet, Benjamin Rotenberg, Maximilien Levesque, Daniel Borgis and Mathieu Salanne
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C8SC04512G
A molecular density functional theory approach to electron transfer reactions
