2019
|
Electroactive fluorescent false neurotransmitter FFN102 partially replaces dopamine in PC12 cell vesicles Article de journal L Hu; A Savy; L Grimaud; M Guille-Collignon; F Lemaître; C Amatore; J Delacotte Biophysical Chemistry, 245 , p. 1–5, 2019. @article{Hu:2019,
title = {Electroactive fluorescent false neurotransmitter FFN102 partially replaces dopamine in PC12 cell vesicles},
author = {L Hu and A Savy and L Grimaud and M Guille-Collignon and F Lema\^{i}tre and C Amatore and J Delacotte},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85057201704&doi=10.1016%2fj.bpc.2018.11.001&partnerID=40&md5=28655b4c152ce0fc51fc037feefcff97},
doi = {10.1016/j.bpc.2018.11.001},
year = {2019},
date = {2019-01-01},
journal = {Biophysical Chemistry},
volume = {245},
pages = {1--5},
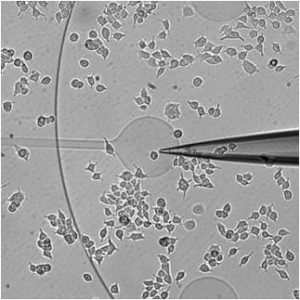
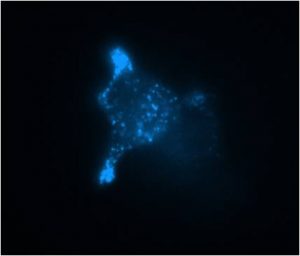
abstract = {In the last decade, following fluorescent dyes and protein tags, pH sensitive false fluorescent neurotransmitters (FFN) were introduced and were valuable for labeling secretory vesicles and monitoring exocytosis at living cells. In particular, the synthetic analog of neurotransmitters FFN102 was shown to be an electroactive probe. Here, we show that FFN102 is suitable to be used as a bioanalytic probe at the widely used PC12 cell model. FFN102 was uptaken in the secretory vesicles of PC12 cells, partially replacing the endogenous dopamine stored in these vesicles. The different oxidation potentials of dopamine and FFN102 allowed to determine that ca. 12% of dopamine was replaced by FFN102. Moreover, the FFN102 was found to be over released through the initial fusion pore suggesting that it was mostly uptaken in fast diffusion compartment of the vesicles. © 2018 Elsevier B.V.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
In the last decade, following fluorescent dyes and protein tags, pH sensitive false fluorescent neurotransmitters (FFN) were introduced and were valuable for labeling secretory vesicles and monitoring exocytosis at living cells. In particular, the synthetic analog of neurotransmitters FFN102 was shown to be an electroactive probe. Here, we show that FFN102 is suitable to be used as a bioanalytic probe at the widely used PC12 cell model. FFN102 was uptaken in the secretory vesicles of PC12 cells, partially replacing the endogenous dopamine stored in these vesicles. The different oxidation potentials of dopamine and FFN102 allowed to determine that ca. 12% of dopamine was replaced by FFN102. Moreover, the FFN102 was found to be over released through the initial fusion pore suggesting that it was mostly uptaken in fast diffusion compartment of the vesicles. © 2018 Elsevier B.V. |
2018
|
Coupling electrochemistry and TIRF-microscopy with the fluorescent false neurotransmitter FFN102 supports the fluorescence signals during single vesicle exocytosis detection Article de journal Xiaoqing Liu; Lihui Hu; Na Pan; Laurence Grimaud; Eric Labbé; Olivier Buriez; Jér^ome Delacotte; Frédéric Lema^itre; Manon Guille-Collignon Biophysical chemistry, 235 , p. 48–55, 2018. @article{liu2018coupling,
title = {Coupling electrochemistry and TIRF-microscopy with the fluorescent false neurotransmitter FFN102 supports the fluorescence signals during single vesicle exocytosis detection},
author = {Xiaoqing Liu and Lihui Hu and Na Pan and Laurence Grimaud and Eric Labb\'{e} and Olivier Buriez and J\'{e}r{^o}me Delacotte and Fr\'{e}d\'{e}ric Lema{^i}tre and Manon Guille-Collignon},
year = {2018},
date = {2018-01-01},
journal = {Biophysical chemistry},
volume = {235},
pages = {48--55},
publisher = {Elsevier},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Coupling electrochemistry and TIRF-microscopy with the fluorescent false neurotransmitter FFN102 supports the fluorescence signals during single vesicle exocytosis detection Article de journal X Liu; L Hu; N Pan; L Grimaud; E Labbé; O Buriez; J Delacotte; F Lemaître; M Guille-Collignon Biophysical Chemistry, 235 , p. 48–55, 2018. @article{Liu:2018,
title = {Coupling electrochemistry and TIRF-microscopy with the fluorescent false neurotransmitter FFN102 supports the fluorescence signals during single vesicle exocytosis detection},
author = {X Liu and L Hu and N Pan and L Grimaud and E Labb\'{e} and O Buriez and J Delacotte and F Lema\^{i}tre and M Guille-Collignon},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85042352158&doi=10.1016%2fj.bpc.2018.02.004&partnerID=40&md5=365430d79a0d526895e755729264d88f},
doi = {10.1016/j.bpc.2018.02.004},
year = {2018},
date = {2018-01-01},
journal = {Biophysical Chemistry},
volume = {235},
pages = {48--55},
abstract = {Applications of the Fluorescent False Neurotransmitter FFN102, an analog of biogenic neurotransmitters and a suitable probe for coupled amperometry and TIRFM (total internal reflexion fluorescence microscopy) investigations of exocytotic secretion, were considered here. The electroactivity of FFN102 was shown to very likely arise from the oxidation of its phenolic group through a CE (Chemical-Electrochemical) mechanism. Evidences that the aminoethyl group of FFN102 is the key recognition element by BON N13 cells were also provided. Amperometric measurements were then performed at the single cell level with carbon fiber electrode (CFE) or Indium Tin Oxide (ITO) surfaces. It proved the disparity of kinetic and quantitative parameters of FFN102-stained cells acquired either at cell top and bottom. Moreover, coupled analyses of FFN102 loaded vesicles allowed us to classify three types of optical signals that probably arise from secretion releases thanks to their concomitant detection with an electrochemical spike. Finally, preliminary benefits from the coupling involving FFN102 were reported in terms of origins of overlapped amperometric spikes or assignment of fluorescence extinctions to real exocytotic events. © 2018 Elsevier B.V.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Applications of the Fluorescent False Neurotransmitter FFN102, an analog of biogenic neurotransmitters and a suitable probe for coupled amperometry and TIRFM (total internal reflexion fluorescence microscopy) investigations of exocytotic secretion, were considered here. The electroactivity of FFN102 was shown to very likely arise from the oxidation of its phenolic group through a CE (Chemical-Electrochemical) mechanism. Evidences that the aminoethyl group of FFN102 is the key recognition element by BON N13 cells were also provided. Amperometric measurements were then performed at the single cell level with carbon fiber electrode (CFE) or Indium Tin Oxide (ITO) surfaces. It proved the disparity of kinetic and quantitative parameters of FFN102-stained cells acquired either at cell top and bottom. Moreover, coupled analyses of FFN102 loaded vesicles allowed us to classify three types of optical signals that probably arise from secretion releases thanks to their concomitant detection with an electrochemical spike. Finally, preliminary benefits from the coupling involving FFN102 were reported in terms of origins of overlapped amperometric spikes or assignment of fluorescence extinctions to real exocytotic events. © 2018 Elsevier B.V. |
Downstream Simultaneous Electrochemical Detection of Primary Reactive Oxygen and Nitrogen Species Released by Cell Populations in an Integrated Microfluidic Device Article de journal Y Li; C Sella; F Lemaître; M Guille-Collignon; C Amatore; L Thouin Analytical Chemistry, 90 (15), p. 9386–9394, 2018. @article{Li:2018,
title = {Downstream Simultaneous Electrochemical Detection of Primary Reactive Oxygen and Nitrogen Species Released by Cell Populations in an Integrated Microfluidic Device},
author = {Y Li and C Sella and F Lema\^{i}tre and M Guille-Collignon and C Amatore and L Thouin},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85049664645&doi=10.1021%2facs.analchem.8b02039&partnerID=40&md5=8f713011165b3b5e06d267ffccea9277},
doi = {10.1021/acs.analchem.8b02039},
year = {2018},
date = {2018-01-01},
journal = {Analytical Chemistry},
volume = {90},
number = {15},
pages = {9386--9394},
abstract = {An innovative microfluidic platform was designed to monitor electrochemically four primary reactive oxygen (ROS) and reactive nitrogen species (RNS) released by aerobic cells. Taking advantage of the space confinement and electrode performances under flow conditions, only a few experiments were sufficient to directly provide significant statistical data relative to the average behavior of cells during oxidative-stress bursts. The microfluidic platform comprised an upstream microchamber for cell culture and four parallel microchannels located downstream for separately detecting H2O2, ONOO-, NO·, and NO2 -. Amperometric measurements were performed at highly sensitive Pt-black electrodes implemented in the microchannels. RAW 264.7 macrophage secretions triggered by a calcium ionophore were used as a way to assess the performance, sensitivity, and specificity of the integrated microfluidic device. In comparison with some previous evaluations achieved from single-cell measurements, reproducible and relevant determinations validated the proof of concept of this microfluidic platform for analyzing statistically significant oxidative-stress responses of various cell types. Copyright © 2018 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
An innovative microfluidic platform was designed to monitor electrochemically four primary reactive oxygen (ROS) and reactive nitrogen species (RNS) released by aerobic cells. Taking advantage of the space confinement and electrode performances under flow conditions, only a few experiments were sufficient to directly provide significant statistical data relative to the average behavior of cells during oxidative-stress bursts. The microfluidic platform comprised an upstream microchamber for cell culture and four parallel microchannels located downstream for separately detecting H2O2, ONOO-, NO·, and NO2 -. Amperometric measurements were performed at highly sensitive Pt-black electrodes implemented in the microchannels. RAW 264.7 macrophage secretions triggered by a calcium ionophore were used as a way to assess the performance, sensitivity, and specificity of the integrated microfluidic device. In comparison with some previous evaluations achieved from single-cell measurements, reproducible and relevant determinations validated the proof of concept of this microfluidic platform for analyzing statistically significant oxidative-stress responses of various cell types. Copyright © 2018 American Chemical Society. |
Investigation of photocurrents resulting from a living unicellular algae suspension with quinones over time Article de journal G Longatte; A Sayegh; J Delacotte; F Rappaport; F -A Wollman; M Guille-Collignon; F Lemaître Chemical Science, 9 (43), p. 8271–8281, 2018. @article{Longatte:2018,
title = {Investigation of photocurrents resulting from a living unicellular algae suspension with quinones over time},
author = {G Longatte and A Sayegh and J Delacotte and F Rappaport and F -A Wollman and M Guille-Collignon and F Lema\^{i}tre},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85056308267&doi=10.1039%2fc8sc03058h&partnerID=40&md5=73d658b7ab313cc1a772ca28dc56aa2d},
doi = {10.1039/c8sc03058h},
year = {2018},
date = {2018-01-01},
journal = {Chemical Science},
volume = {9},
number = {43},
pages = {8271--8281},
abstract = {Plants, algae, and some bacteria convert solar energy into chemical energy by using photosynthesis. In light of the current energy environment, many research strategies try to benefit from photosynthesis in order to generate usable photobioelectricity. Among all the strategies developed for transferring electrons from the photosynthetic chain to an outer collecting electrode, we recently implemented a method on a preparative scale (high surface electrode) based on a Chlamydomonas reinhardtii green algae suspension in the presence of exogenous quinones as redox mediators. While giving rise to an interesting performance (10-60 μA cm-2) in the course of one hour, this device appears to cause a slow decrease of the recorded photocurrent. In this paper, we wish to analyze and understand this gradual fall in performance in order to limit this issue in future applications. We thus first show that this kind of degradation could be related to over-irradiation conditions or side-effects of quinones depending on experimental conditions. We therefore built an empirical model involving a kinetic quenching induced by incubation with quinones, which is globally consistent with the experimental data provided by fluorescence measurements achieved after dark incubation of algae in the presence of quinones. © 2018 The Royal Society of Chemistry.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Plants, algae, and some bacteria convert solar energy into chemical energy by using photosynthesis. In light of the current energy environment, many research strategies try to benefit from photosynthesis in order to generate usable photobioelectricity. Among all the strategies developed for transferring electrons from the photosynthetic chain to an outer collecting electrode, we recently implemented a method on a preparative scale (high surface electrode) based on a Chlamydomonas reinhardtii green algae suspension in the presence of exogenous quinones as redox mediators. While giving rise to an interesting performance (10-60 μA cm-2) in the course of one hour, this device appears to cause a slow decrease of the recorded photocurrent. In this paper, we wish to analyze and understand this gradual fall in performance in order to limit this issue in future applications. We thus first show that this kind of degradation could be related to over-irradiation conditions or side-effects of quinones depending on experimental conditions. We therefore built an empirical model involving a kinetic quenching induced by incubation with quinones, which is globally consistent with the experimental data provided by fluorescence measurements achieved after dark incubation of algae in the presence of quinones. © 2018 The Royal Society of Chemistry. |
Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells Article de journal Martina Č'ižková; Laurent Cattiaux; Justine Pandard; Manon Guille-Collignon; Frédéric Lema^itre; Jér^ome Delacotte; Jean-Maurice Mallet; Eric Labbé; Olivier Buriez Electrochemistry Communications, 97 , p. 46–50, 2018. @article{vcivzkova2018redox,
title = {Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells},
author = {Martina {\v{C}}{'i}{\v{z}}kov\'{a} and Laurent Cattiaux and Justine Pandard and Manon Guille-Collignon and Fr\'{e}d\'{e}ric Lema{^i}tre and J\'{e}r{^o}me Delacotte and Jean-Maurice Mallet and Eric Labb\'{e} and Olivier Buriez},
year = {2018},
date = {2018-01-01},
journal = {Electrochemistry Communications},
volume = {97},
pages = {46--50},
publisher = {Elsevier},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells Article de journal M Čížková; L Cattiaux; J Pandard; M Guille-Collignon; F Lemaître; J Delacotte; J -M Mallet; E Labbé; O Buriez Electrochemistry Communications, 97 , p. 46–50, 2018. @article{Cizkova:2018,
title = {Redox switchable rhodamine-ferrocene dyad: Exploring imaging possibilities in cells},
author = {M \v{C}\'{i}\v{z}kov\'{a} and L Cattiaux and J Pandard and M Guille-Collignon and F Lema\^{i}tre and J Delacotte and J -M Mallet and E Labb\'{e} and O Buriez},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85054592456&doi=10.1016%2fj.elecom.2018.10.009&partnerID=40&md5=10a4aed1c89bb6a788a2a260bbd0a818},
doi = {10.1016/j.elecom.2018.10.009},
year = {2018},
date = {2018-01-01},
journal = {Electrochemistry Communications},
volume = {97},
pages = {46--50},
abstract = {An original redox-responsive fluorescent probe combining a rhodamine derivative and a ferrocenyl moiety used as the fluorescence modulator was designed, synthesized and characterized. The fluorescence of this new dyad could be tuned from the redox state of ferrocene, a feature observed both electrochemically and on cancer cells incubated with this probe. © 2018 Elsevier B.V.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
An original redox-responsive fluorescent probe combining a rhodamine derivative and a ferrocenyl moiety used as the fluorescence modulator was designed, synthesized and characterized. The fluorescence of this new dyad could be tuned from the redox state of ferrocene, a feature observed both electrochemically and on cancer cells incubated with this probe. © 2018 Elsevier B.V. |
2017
|
A Dual Functional Electroactive and Fluorescent Probe for Coupled Measurements of Vesicular Exocytosis with High Spatial and Temporal Resolution Article de journal X Liu; A Savy; S Maurin; L Grimaud; F Darchen; D Quinton; E Labbé; O Buriez; J Delacotte; F Lemaître; M Guille-Collignon Angewandte Chemie - International Edition, 56 (9), p. 2366–2370, 2017. @article{Liu:2017a,
title = {A Dual Functional Electroactive and Fluorescent Probe for Coupled Measurements of Vesicular Exocytosis with High Spatial and Temporal Resolution},
author = {X Liu and A Savy and S Maurin and L Grimaud and F Darchen and D Quinton and E Labb\'{e} and O Buriez and J Delacotte and F Lema\^{i}tre and M Guille-Collignon},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85010696856&doi=10.1002%2fanie.201611145&partnerID=40&md5=a51767157166d7f185f0195a28b347b8},
doi = {10.1002/anie.201611145},
year = {2017},
date = {2017-01-01},
journal = {Angewandte Chemie - International Edition},
volume = {56},
number = {9},
pages = {2366--2370},
abstract = {In this work, Fluorescent False Neurotransmitter 102 (FFN102), a synthesized analogue of biogenic neurotransmitters, was demonstrated to show both pH-dependent fluorescence and electroactivity. To study secretory behaviors at the single-vesicle level, FFN102 was employed as a new fluorescent/electroactive dual probe in a coupled technique (amperometry and total internal reflection fluorescence microscopy (TIRFM)). We used N13 cells, a stable clone of BON cells, to specifically accumulate FFN102 into their secretory vesicles, and then optical and electrochemical measurements of vesicular exocytosis were experimentally achieved by using indium tin oxide (ITO) transparent electrodes. Upon stimulation, FFN102 started to diffuse out from the acidic intravesicular microenvironment to the neutral extracellular space, leading to fluorescent emissions and to the electrochemical oxidation signals that were simultaneously collected from the ITO electrode surface. The correlation of fluorescence and amperometric signals resulting from the FFN102 probe allows real-time monitoring of single exocytotic events with both high spatial and temporal resolution. This work opens new possibilities in the investigation of exocytotic mechanisms. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
In this work, Fluorescent False Neurotransmitter 102 (FFN102), a synthesized analogue of biogenic neurotransmitters, was demonstrated to show both pH-dependent fluorescence and electroactivity. To study secretory behaviors at the single-vesicle level, FFN102 was employed as a new fluorescent/electroactive dual probe in a coupled technique (amperometry and total internal reflection fluorescence microscopy (TIRFM)). We used N13 cells, a stable clone of BON cells, to specifically accumulate FFN102 into their secretory vesicles, and then optical and electrochemical measurements of vesicular exocytosis were experimentally achieved by using indium tin oxide (ITO) transparent electrodes. Upon stimulation, FFN102 started to diffuse out from the acidic intravesicular microenvironment to the neutral extracellular space, leading to fluorescent emissions and to the electrochemical oxidation signals that were simultaneously collected from the ITO electrode surface. The correlation of fluorescence and amperometric signals resulting from the FFN102 probe allows real-time monitoring of single exocytotic events with both high spatial and temporal resolution. This work opens new possibilities in the investigation of exocytotic mechanisms. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim |
Electrocatalytic Mechanism Involving Michaelis–Menten Kinetics at the Preparative Scale: Theory and Applicability to Photocurrents from a Photosynthetic Algae Suspension With Quinones Article de journal G Longatte; M Guille-Collignon; F Lemaître ChemPhysChem, 18 (19), p. 2643–2650, 2017. @article{Longatte:2017a,
title = {Electrocatalytic Mechanism Involving Michaelis\textendashMenten Kinetics at the Preparative Scale: Theory and Applicability to Photocurrents from a Photosynthetic Algae Suspension With Quinones},
author = {G Longatte and M Guille-Collignon and F Lema\^{i}tre},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85028605961&doi=10.1002%2fcphc.201700351&partnerID=40&md5=0500ecaa88d980132140883a725bedfb},
doi = {10.1002/cphc.201700351},
year = {2017},
date = {2017-01-01},
journal = {ChemPhysChem},
volume = {18},
number = {19},
pages = {2643--2650},
abstract = {In the past years, many strategies have been implemented to benefit from oxygenic photosynthesis to harvest photosynthetic electrons and produce a significant photocurrent. Therefore, electrochemical tools were considered and have globally relied on the electron transfer(s) between the photosynthetic chain and a collecting electrode. In this context, we recently reported the implementation of an electrochemical set-up at the preparative scale to produce photocurrents from a Chlamydomonas reinhardtii algae suspension with an appropriate mediator (2,6-DCBQ) and a carbon gauze as the working electrode. In the present work, we wish to describe a mathematical modeling of the recorded photocurrents to better understand the effects of the experimental conditions on the photosynthetic extraction of electrons. In that way, we established a general model of an electrocatalytic mechanism at the preparative scale (that is, assuming a homogenous bulk solution at any time and a constant diffusion layer, both assumptions being valid under forced convection) in which the chemical step involves a Michaelis\textendashMenten-like behaviour. Dependences of transient and steady-state corresponding currents were analysed as a function of different parameters by means of zone diagrams. This model was tested to our experimental data related to photosynthesis. The corresponding results suggest that competitive pathways beyond photosynthetic harvesting alone should be taken into account. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
In the past years, many strategies have been implemented to benefit from oxygenic photosynthesis to harvest photosynthetic electrons and produce a significant photocurrent. Therefore, electrochemical tools were considered and have globally relied on the electron transfer(s) between the photosynthetic chain and a collecting electrode. In this context, we recently reported the implementation of an electrochemical set-up at the preparative scale to produce photocurrents from a Chlamydomonas reinhardtii algae suspension with an appropriate mediator (2,6-DCBQ) and a carbon gauze as the working electrode. In the present work, we wish to describe a mathematical modeling of the recorded photocurrents to better understand the effects of the experimental conditions on the photosynthetic extraction of electrons. In that way, we established a general model of an electrocatalytic mechanism at the preparative scale (that is, assuming a homogenous bulk solution at any time and a constant diffusion layer, both assumptions being valid under forced convection) in which the chemical step involves a Michaelis–Menten-like behaviour. Dependences of transient and steady-state corresponding currents were analysed as a function of different parameters by means of zone diagrams. This model was tested to our experimental data related to photosynthesis. The corresponding results suggest that competitive pathways beyond photosynthetic harvesting alone should be taken into account. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim |
Electrochemical Harvesting of Photosynthetic Electrons from Unicellular Algae Population at the Preparative Scale by Using 2,6-dichlorobenzoquinone Article de journal G Longatte; F Rappaport; F -A Wollman; M Guille-Collignon; F Lemaître Electrochimica Acta, 236 , p. 337–342, 2017. @article{Longatte:2017,
title = {Electrochemical Harvesting of Photosynthetic Electrons from Unicellular Algae Population at the Preparative Scale by Using 2,6-dichlorobenzoquinone},
author = {G Longatte and F Rappaport and F -A Wollman and M Guille-Collignon and F Lema\^{i}tre},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85016504359&doi=10.1016%2fj.electacta.2017.03.124&partnerID=40&md5=d8a7614ce4f287a9f115d922ab5ee8f6},
doi = {10.1016/j.electacta.2017.03.124},
year = {2017},
date = {2017-01-01},
journal = {Electrochimica Acta},
volume = {236},
pages = {337--342},
abstract = {Oxygenic photosynthesis is the process used by plants, cyanobacteria or algae to convert the solar energy into a chemical one from the carbon dioxide reduction and water oxidation. In the past years, many strategies were implemented to take benefits from the overall low yield of this process to extract photosynthetic electrons and thus produce a sustainable photocurrent. In practice, electrochemical tools were involved and the principle of electrons harvestings was related to the step of electron transfer between the photosynthetic organism and a collecting electrode. In this context, works involving an algae population in suspension were rather scarce and rather focus on the grafting of the photosynthetic machinery at the electrode surface. Based on our previous works, we report here the implementation of an electrochemical set-up at the preparative scale to produce photocurrents. An algae suspension, i.e. an intact biological system to ensure culture and growth, was involved in presence of a centimeter-sized carbon gauze as the collecting electrode. The spectroelectrochemical cell contains 16 mL of suspension of a Chlamydomonas reinhardtii mutant with an appropriate mediator (2,6-DCBQ). Under these conditions, stable photocurrents were recorded over 1 h whose magnitude depends on the quinone concentration and the light illumination. © 2017 Elsevier Ltd},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Oxygenic photosynthesis is the process used by plants, cyanobacteria or algae to convert the solar energy into a chemical one from the carbon dioxide reduction and water oxidation. In the past years, many strategies were implemented to take benefits from the overall low yield of this process to extract photosynthetic electrons and thus produce a sustainable photocurrent. In practice, electrochemical tools were involved and the principle of electrons harvestings was related to the step of electron transfer between the photosynthetic organism and a collecting electrode. In this context, works involving an algae population in suspension were rather scarce and rather focus on the grafting of the photosynthetic machinery at the electrode surface. Based on our previous works, we report here the implementation of an electrochemical set-up at the preparative scale to produce photocurrents. An algae suspension, i.e. an intact biological system to ensure culture and growth, was involved in presence of a centimeter-sized carbon gauze as the collecting electrode. The spectroelectrochemical cell contains 16 mL of suspension of a Chlamydomonas reinhardtii mutant with an appropriate mediator (2,6-DCBQ). Under these conditions, stable photocurrents were recorded over 1 h whose magnitude depends on the quinone concentration and the light illumination. © 2017 Elsevier Ltd |
Indium Tin Oxide Microsystem for Electrochemical Detection of Exocytosis of Migratory Dendritic Cells Article de journal X Liu; M Bretou; A -M Lennon-Duménil; F Lemaître; M Guille-Collignon Electroanalysis, 29 (1), p. 197–202, 2017. @article{Liu:2017b,
title = {Indium Tin Oxide Microsystem for Electrochemical Detection of Exocytosis of Migratory Dendritic Cells},
author = {X Liu and M Bretou and A -M Lennon-Dum\'{e}nil and F Lema\^{i}tre and M Guille-Collignon},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-84992456261&doi=10.1002%2felan.201600360&partnerID=40&md5=dbe0bcd2017bc2da52f267117aab382f},
doi = {10.1002/elan.201600360},
year = {2017},
date = {2017-01-01},
journal = {Electroanalysis},
volume = {29},
number = {1},
pages = {197--202},
abstract = {The design, fabrication and test of an indium tin oxide (ITO) microdevice to investigate exocytotic behaviors of migratory dendritic cells (DCs) in confined three-dimensional environment were reported in this work. Indeed, immature DCs were able to migrate into micro-fabricated biocompatible polydimethylsiloxane (PDMS) channels that mimic their natural constrained environment of tissues for patrolling in search of danger associated antigens through an endocytotic process called macropinocytosis. In order to coordinate membrane trafficking and prevent cell volume increment, DCs will release part of their contents back to the extracellular medium while migrating. Through electrochemical measurements, we demonstrated that exocytotic events of migratory DCs could be monitored by our ITO microdevice. In addition, the transparency of ITO electrode should facilitate future combining assays of exocytosis with other fluorescence-based measurements of cell physiology. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The design, fabrication and test of an indium tin oxide (ITO) microdevice to investigate exocytotic behaviors of migratory dendritic cells (DCs) in confined three-dimensional environment were reported in this work. Indeed, immature DCs were able to migrate into micro-fabricated biocompatible polydimethylsiloxane (PDMS) channels that mimic their natural constrained environment of tissues for patrolling in search of danger associated antigens through an endocytotic process called macropinocytosis. In order to coordinate membrane trafficking and prevent cell volume increment, DCs will release part of their contents back to the extracellular medium while migrating. Through electrochemical measurements, we demonstrated that exocytotic events of migratory DCs could be monitored by our ITO microdevice. In addition, the transparency of ITO electrode should facilitate future combining assays of exocytosis with other fluorescence-based measurements of cell physiology. © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim |
Redesigning the QA binding site of Photosystem II allows reduction of exogenous quinones Article de journal H -Y Fu; D Picot; Y Choquet; G Longatte; A Sayegh; J Delacotte; M Guille-Collignon; F Lemaýtre; F Rappaport; F -A Wollman Nature Communications, 8 , 2017. @article{Fu:2017,
title = {Redesigning the QA binding site of Photosystem II allows reduction of exogenous quinones},
author = {H -Y Fu and D Picot and Y Choquet and G Longatte and A Sayegh and J Delacotte and M Guille-Collignon and F Lema\'{y}tre and F Rappaport and F -A Wollman},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-85030692080&doi=10.1038%2fncomms15274&partnerID=40&md5=d602745399324d5a5eb6c25bd2e5a7c7},
doi = {10.1038/ncomms15274},
year = {2017},
date = {2017-01-01},
journal = {Nature Communications},
volume = {8},
abstract = {Strategies to harness photosynthesis from living organisms to generate electrical power have long been considered, yet efficiency remains low. Here, we aimed to reroute photosynthetic electron flow in photosynthetic organisms without compromising their phototrophic properties. We show that 2,6-dimethyl-p-benzoquinone (DMBQ) can be used as an electron mediator to assess the efficiency of mutations designed to engineer a novel electron donation pathway downstream of the primary electron acceptor QA of Photosystem (PS) II in the green alga Chlamydomonas reinhardtii. Through the use of structural prediction studies and a screen of site-directed PSII mutants we show that modifying the environment of the QA site increases the reduction rate of DMBQ. Truncating the C-terminus of the PsbT subunit protruding in the stroma provides evidence that shortening the distance between QA and DMBQ leads to sustained electron transfer to DMBQ, as confirmed by chronoamperometry, consistent with a bypass of the natural QA7circ; to QB pathway.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Strategies to harness photosynthesis from living organisms to generate electrical power have long been considered, yet efficiency remains low. Here, we aimed to reroute photosynthetic electron flow in photosynthetic organisms without compromising their phototrophic properties. We show that 2,6-dimethyl-p-benzoquinone (DMBQ) can be used as an electron mediator to assess the efficiency of mutations designed to engineer a novel electron donation pathway downstream of the primary electron acceptor QA of Photosystem (PS) II in the green alga Chlamydomonas reinhardtii. Through the use of structural prediction studies and a screen of site-directed PSII mutants we show that modifying the environment of the QA site increases the reduction rate of DMBQ. Truncating the C-terminus of the PsbT subunit protruding in the stroma provides evidence that shortening the distance between QA and DMBQ leads to sustained electron transfer to DMBQ, as confirmed by chronoamperometry, consistent with a bypass of the natural QA7circ; to QB pathway. |
2016
|
Astrocyte-derived adenosine is central to the hypnogenic effect of glucose Article de journal E Scharbarg; M Daenens; F Lemaître; H Geoffroy; M Guille-Collignon; T Gallopin; A Rancillac Scientific Reports, 6 , 2016. @article{Scharbarg:2016,
title = {Astrocyte-derived adenosine is central to the hypnogenic effect of glucose},
author = {E Scharbarg and M Daenens and F Lema\^{i}tre and H Geoffroy and M Guille-Collignon and T Gallopin and A Rancillac},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-84954456013&doi=10.1038%2fsrep19107&partnerID=40&md5=e785b2e364698aba76cea9c2cade445e},
doi = {10.1038/srep19107},
year = {2016},
date = {2016-01-01},
journal = {Scientific Reports},
volume = {6},
abstract = {Sleep has been hypothesised to maintain a close relationship with metabolism. Here we focus on the brain structure that triggers slow-wave sleep, the ventrolateral preoptic nucleus (VLPO), to explore the cellular and molecular signalling pathways recruited by an increase in glucose concentration. We used infrared videomicroscopy on ex vivo brain slices to establish that glucose induces vasodilations specifically in the VLPO via the astrocytic release of adenosine. Real-time detection by in situ purine biosensors further revealed that the adenosine level doubles in response to glucose, and triples during the wakefulness period. Finally, patch-clamp recordings uncovered the depolarizing effect of adenosine and its A2A receptor agonist, CGS-21680, on sleep-promoting VLPO neurons. Altogether, our results provide new insights into the metabolically driven release of adenosine. We hypothesise that adenosine adjusts the local energy supply to local neuronal activity in response to glucose. This pathway could contribute to sleep-wake transition and sleep intensity.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Sleep has been hypothesised to maintain a close relationship with metabolism. Here we focus on the brain structure that triggers slow-wave sleep, the ventrolateral preoptic nucleus (VLPO), to explore the cellular and molecular signalling pathways recruited by an increase in glucose concentration. We used infrared videomicroscopy on ex vivo brain slices to establish that glucose induces vasodilations specifically in the VLPO via the astrocytic release of adenosine. Real-time detection by in situ purine biosensors further revealed that the adenosine level doubles in response to glucose, and triples during the wakefulness period. Finally, patch-clamp recordings uncovered the depolarizing effect of adenosine and its A2A receptor agonist, CGS-21680, on sleep-promoting VLPO neurons. Altogether, our results provide new insights into the metabolically driven release of adenosine. We hypothesise that adenosine adjusts the local energy supply to local neuronal activity in response to glucose. This pathway could contribute to sleep-wake transition and sleep intensity. |
Mechanism and analyses for extracting photosynthetic electrons using exogenous quinones-what makes a good extraction pathway? Article de journal G Longatte; F Rappaport; F -A Wollman; M Guille-Collignon; F Lemaître Photochemical and Photobiological Sciences, 15 (8), p. 969–979, 2016. @article{Longatte:2016,
title = {Mechanism and analyses for extracting photosynthetic electrons using exogenous quinones-what makes a good extraction pathway?},
author = {G Longatte and F Rappaport and F -A Wollman and M Guille-Collignon and F Lema\^{i}tre},
url = {https://www.scopus.com/inward/record.uri?eid=2-s2.0-84982703184&doi=10.1039%2fc6pp00076b&partnerID=40&md5=bf03d01f7c5b19ff550d5b9f76d49eb9},
doi = {10.1039/c6pp00076b},
year = {2016},
date = {2016-01-01},
journal = {Photochemical and Photobiological Sciences},
volume = {15},
number = {8},
pages = {969--979},
abstract = {Plants or algae take many benefits from oxygenic photosynthesis by converting solar energy into chemical energy through the synthesis of carbohydrates from carbon dioxide and water. However, the overall yield of this process is rather low (about 4% of the total energy available from sunlight is converted into chemical energy). This is the principal reason why recently many studies have been devoted to extraction of photosynthetic electrons in order to produce a sustainable electric current. Practically, the electron transfer occurs between the photosynthetic organism and an electrode and can be assisted by an exogenous mediator, mainly a quinone. In this regard, we recently reported on a method involving fluorescence measurements to estimate the ability of different quinones to extract photosynthetic electrons from a mutant of Chlamydomonas reinhardtii. In the present work, we used the same kind of methodology to establish a zone diagram for predicting the most suitable experimental conditions to extract photoelectrons from intact algae (quinone concentration and light intensity) as a function of the purpose of the study. This will provide further insights into the extraction mechanism of photosynthetic electrons using exogenous quinones. Indeed fluorescence measurements allowed us to model the capacity of photosynthetic algae to donate electrons to an exogenous quinone by considering a numerical parameter called "open center ratio" which is related to the Photosystem II acceptor redox state. Then, using it as a proxy for investigating the extraction of photosynthetic electrons by means of an exogenous quinone, 2,6-DCBQ, we suggested an extraction mechanism that was globally found consistent with the experimentally extracted parameters. © The Royal Society of Chemistry and Owner Societies 2016.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Plants or algae take many benefits from oxygenic photosynthesis by converting solar energy into chemical energy through the synthesis of carbohydrates from carbon dioxide and water. However, the overall yield of this process is rather low (about 4% of the total energy available from sunlight is converted into chemical energy). This is the principal reason why recently many studies have been devoted to extraction of photosynthetic electrons in order to produce a sustainable electric current. Practically, the electron transfer occurs between the photosynthetic organism and an electrode and can be assisted by an exogenous mediator, mainly a quinone. In this regard, we recently reported on a method involving fluorescence measurements to estimate the ability of different quinones to extract photosynthetic electrons from a mutant of Chlamydomonas reinhardtii. In the present work, we used the same kind of methodology to establish a zone diagram for predicting the most suitable experimental conditions to extract photoelectrons from intact algae (quinone concentration and light intensity) as a function of the purpose of the study. This will provide further insights into the extraction mechanism of photosynthetic electrons using exogenous quinones. Indeed fluorescence measurements allowed us to model the capacity of photosynthetic algae to donate electrons to an exogenous quinone by considering a numerical parameter called "open center ratio" which is related to the Photosystem II acceptor redox state. Then, using it as a proxy for investigating the extraction of photosynthetic electrons by means of an exogenous quinone, 2,6-DCBQ, we suggested an extraction mechanism that was globally found consistent with the experimentally extracted parameters. © The Royal Society of Chemistry and Owner Societies 2016. |
More Transparency in BioAnalysis of Exocytosis: Coupling of Electrochemistry and Fluorescence Microscopy at ITO Electrodes Book Chapter Xiaoqing Liu; Damien Quinton; Lihui Hu; Christian Amatore; Jerome Delacotte; Frederic Lemaitre; Manon Guille-Collignon Raspaud, E; Marliere, C; Regeard, C; Cornut, R; MealletRenault, R (Ed.): Electro-Activity of Biological Systems, 6 , 2016, (Times Cited: 0
International and Multidisciplinary Workshop on Electro-Activity of Biological Systems (EABS)
Nov 18-19, 2015
Paris, FRANCE). @inbook{,
title = {More Transparency in BioAnalysis of Exocytosis: Coupling of Electrochemistry and Fluorescence Microscopy at ITO Electrodes},
author = {Xiaoqing Liu and Damien Quinton and Lihui Hu and Christian Amatore and Jerome Delacotte and Frederic Lemaitre and Manon Guille-Collignon},
editor = {E Raspaud and C Marliere and C Regeard and R Cornut and R MealletRenault},
year = {2016},
date = {2016-01-01},
booktitle = {Electro-Activity of Biological Systems},
volume = {6},
series = {BIO Web of Conferences},
note = {Times Cited: 0
International and Multidisciplinary Workshop on Electro-Activity of Biological Systems (EABS)
Nov 18-19, 2015
Paris, FRANCE},
keywords = {},
pubstate = {published},
tppubtype = {inbook}
}
|