published in Angew. Chem. Int. Ed., 2022
https://onlinelibrary.wiley.com/doi/10.1002/anie.202203066
1
Metal complexes are increasingly used for biological and medicinal applications, for instance as metal-based drugs (metallodrugs). Biological environments are rich in Lewis bases and contain a variety of divalent metal ions that constitute an exchangeable pool. Exogenous metal complexes can therefore experience metal or ligand exchange in such biological environments. These exchanges decrease their intracellular concentration and/or can change the nature of the active species. Knowing the nature (or speciation) in cellular context is thus key to understand and possibly rationalize the observed biological activity of such complexes.
Mn(II) complexes are particularly difficult to detect and quantify because of the low association constants of ligand for Mn(II), a d5 ion showing no ligand field stabilization energy (LFSE), high ligand lability, and fast metal exchange. The knowledge of the speciation of a Mn(II)-complex or Mn(II) ion in a biological environment is therefore both challenging and crucial.
In the Laboratoire des BioMolécules (ENS-PSL, PSL university, Sorbonne university, CNRS UMR 7203), the multidisciplinary group “Metals in biology and redox homeostasis” develops bioinspired metal-based catalytic antioxidants, namely manganese(II) complexes that mimic superoxide dismutases (SODs). These metalloenzymes catalyze the dismutation of superoxide, a reactive oxygen species (ROS) derived from O2, and constitute the first line of cellular antioxidant defense. The SOD mimic labelled Mn1 (see figure) has good intrinsic anti-superoxide, antioxidant and anti-inflammatory activities in lipopolysaccharide (LPS)-activated intestinal epithelial HT29-MD2 cells. This cellular model is developed in collaboration with the group Microbiote, Intestin et Inflammation from the Centre de Recherche de l’hôpital Saint-Antoine (UMRS 938, SU/INSERM).
The quantification methods previously used (EPR, ICP-MS, XRF) were focused on the metal ion cellular content and its comparison with the endogenous content, assuming that excess of Mn was due to internalized Mn1.
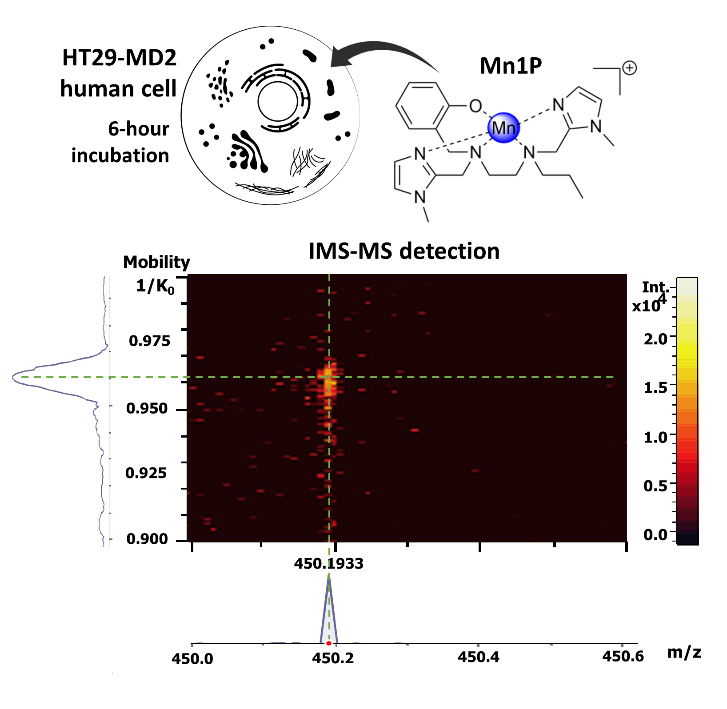
The study, recently published as a Very Important Publication in Angewandte Chemie, Int. Ed., aimed to elucidate the fate of Mn1 and its propylated analogue labelled Mn1P (that showed similar activity to Mn1) in HT29-MD2 cell lysates using direct detection of the molecular species Mn1 and Mn1P based on mass spectrometry (MS).
The group of bioinorganic chemists of the LBM collaborated with the Institute of Analytical Sciences and Physical Chemistry for the Environment and Materials (IPREM) in Pau and the laboratory of biological mass spectrometry and proteomics (SMBP, ESPCI-PSL).
Because of the complexity of the biological media in the masses range of the studied complexes (low-molecular weight complexes), it appeared necessary to couple MS with a separation method. The classical techniques of LC-MS (liquid chromatography coupled to mass spectrometry) proved to be not effective: the authors showed that metallic exchanges occurred in the analytical system. They then considered a coupling with ion mobility. Ion mobility spectrometry (IMS), first developed by Cohen and Karasek in 1970 to detect traces of organic molecules in the gas phase, involves the movement of ions against a gas flow under an applied electric field. In the present work, with a timsTOF, the set-up is slightly different, the metal complexes are “pushed” by the gas flow and “retained” by the electric field. The velocity of ions is proportional to their reduced mobility constant K (cm-1V-1s-1), which is associated with their size and more specifically with their rotationally averaged collision cross-section (CCS, in Å2).
The authors show here that ion mobility is powerful enough to separate complexes of the same ligand bound to different divalent metal cations that nevertheless have similar ionic radii (typically Mn(II), Fe(II), Co(II), Cu(II), Zn(II)).
They apply it, coupled to MS, to demonstrate the intracellular presence of Mn1 and Mn1P complexes, at least partially intact, in lysates from cells incubated with the complexes. Even though they experimentally demonstrate exchange with cellular endogenous metal ions, the complexes were detected. In the case of Mn1P, using a calibration curve involving a heavy analogue (Co-13C6-ligand), they were able to estimate the Mn1P content in the cells at 0.013 ± 0.006 fmol/cell.

See also: https://www.inc.cnrs.fr/fr/cnrsinfo/des-complexes-bio-inspires-dans-le-vent
Source :
Deciphering the Metal Speciation in Low-Molecular-Weight Complexes by IMS-MS: Application to the Detection of Manganese Superoxide Dismutase Mimics in Cell Lysates
Martha Zoumpoulaki, Gabrielle Schanne, Nicolas Delsuc, Hugues Preud’homme, Elodie Quévrain, Nicolas Eskenazi, Géraldine Gazzah, Regis Guillot, Philippe Seksik, Joelle Vinh, Ryszard Lobinski, Clotilde Policar*
[*] Prof. C. Policar, Laboratoire des biomolécules (LBM), Département de chimie, École normale supérieure, PSL University, Sorbonne Université, CNRS, 75005 Paris, France, email : clotilde.policar@ens.psl.eu
Dr. M. Zoumpoulaki, G. Schanne, Dr. N. Delsuc, Laboratoire des biomolécules (LBM), Département de chimie, École normale supérieure, PSL University, Sorbonne Université, CNRS, 75005 Paris, France
Dr. M. Zoumpoulaki, Dr. N. Eskenazi, Dr. J. Vinh, SMBP ESPCI Paris, PSL University, UMR 8249 CNRS
Dr. M. Zoumpoulaki, G. Schanne, Prof./MD P. Seksik, Centre de Recherche de Saint-Antoine, Sorbonne University, INSERM, 75012 Paris (France)
Dr. H. Preud’homme, Prof. R. Lobinski, IPREM-UMR5254, E2S UPPA, CNRS, Technopôle Helioparc, 64053 Pau Cedex 9 (France)
Dr. R. Guillot, Université Paris-Saclay, CNRS, 91405 Orsay (France)
