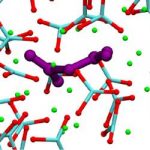
The reactivity, speciation and solvation structure of CO2 in carbonate melts are relevant both for the fate of carbon in deep geological formations and for its electroreduction to CO, to be used as fuel, by means of solvation in a molten carbonate electrolyte. In particular, the high solubility of CO2 in carbonate melts has been tentatively attributed to the formation of a new carbon species, the pyrocarbonate anion, C2O52-. In this work we study, by _rst principles molecular dynamics simulations, the behaviour of CO2 in molten calcium carbonate. We _nd that pyrocarbonate forms spontaneously and the identity of the CO2 molecule is quickly lost through O2- exchange. The transport of CO2 in this molten carbonate thus occurs in a fashion similar to the Grotthuss mechanism in water, and is three times faster than molecular diffusion. This shows that Grotthuss- like transport is more general than thought so far.

N’hésitez pas à consulter le communiqué de presse associé à cet article : Un étonnant mécanisme de diffusion de CO2 dans les carbonates fondus
References:
Carbon dioxide transport in molten calcium carbonate occurs through an oxo-Grotthuss mechanism via a pyrocarbonate anion
Dario Corradini, François-Xavier Coudert, and Rodolphe Vuilleumier
Nature Chemistry, 2016, Feb.
doi:10.1038/nchem.2450
Carbon dioxide transport in molten calcium carbonate occurs through an oxo-Grotthuss mechanism via a pyrocarbonate anion
